
The number of bridging $CO$ ligand (s) and $Co - Co$ bond (s) in $C{o_2}{(CO)_8}$ , respectively are:
a.) 0 and 2
b.) 2 and 0
c.) 4 and 0
d.) 2 and 1
Answer
558.9k+ views
Hint: Spanning ligands can be fundamentally classified as ligands that are associating or joining or at least two iotas/atoms together. These ligands are generally used to interface metal particles. Presently, these ligands may either be mono - nuclear or polyatomic in nature.
Complete step by step answer:
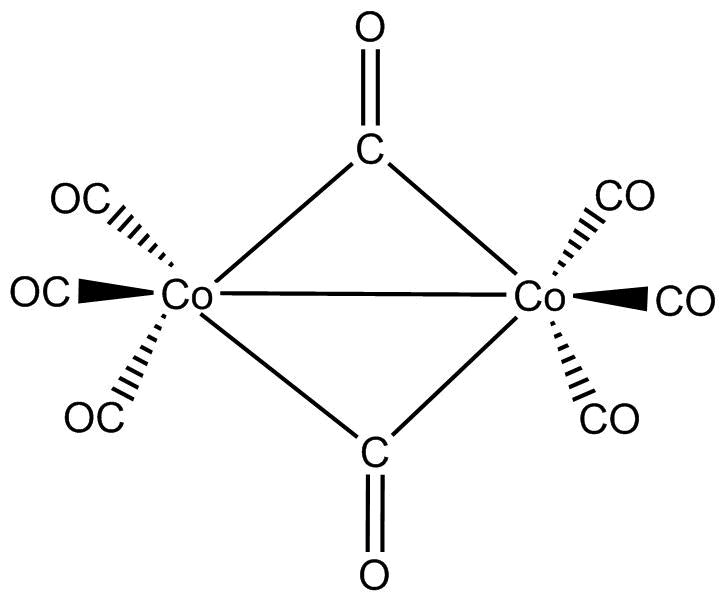
As can be seen from the structure of $C{o_2}{(CO)_8}$, there are two $CO$ crossing over ligands and one $Co - Co$ (Metal-Metal) bond.
Number of $M - M$ Bonds = $\dfrac{{18 \times n - VE}}{2}$
n = number of metals
(i) $C{o_2}{(CO)_8}$
$VE = 9 \times 2 + 2 \times 8$
$ \Rightarrow VE = 34$
Number of $M - M$ Bonds = $\dfrac{{18 \times 2 - 34}}{2}$ = 1
So the number of $Co - Co$ bond is 1.
Verification:
$C{o_2}{(CO)_8}$ is Octahedral in nature
So, the number of ligands in $C{o_2}{(CO)_8}$ are 6.
So, if we re draw the above diagram we would get 2 bridging $CO$ ligands in $C{o_2}{(CO)_8}$
The correct answer is option “D” .
Additional Information :
Bridging ligands: A bridging ligand is a ligand that interfaces at least two molecules, normally metal particles. The ligand might be nuclear or polyatomic. Essentially all intricate natural mixes can fill in as bridging ligands, so the term is normally limited to little ligands, for example, pseudo-halides or to ligands that are explicitly intended to connect two metals.
Note: In naming a complex wherein a solitary particle spans two metals, the connecting ligand is gone before by the Greek letter mu, $\mu $, with a superscript number indicating the quantity of metals bound to the crossing over the ligand. ${\mu _2}$ is frequently signified basically as $\mu $. While depicting coordination buildings care ought to be taken not to mistake $\mu $ for $\eta $, which identifies with hapticity. Ligands that are not connecting are called terminal ligands.
Complete step by step answer:
As can be seen from the structure of $C{o_2}{(CO)_8}$, there are two $CO$ crossing over ligands and one $Co - Co$ (Metal-Metal) bond.
Number of $M - M$ Bonds = $\dfrac{{18 \times n - VE}}{2}$
n = number of metals
(i) $C{o_2}{(CO)_8}$
$VE = 9 \times 2 + 2 \times 8$
$ \Rightarrow VE = 34$
Number of $M - M$ Bonds = $\dfrac{{18 \times 2 - 34}}{2}$ = 1
So the number of $Co - Co$ bond is 1.
Verification:
$C{o_2}{(CO)_8}$ is Octahedral in nature
So, the number of ligands in $C{o_2}{(CO)_8}$ are 6.

So, if we re draw the above diagram we would get 2 bridging $CO$ ligands in $C{o_2}{(CO)_8}$

The correct answer is option “D” .
Additional Information :
Bridging ligands: A bridging ligand is a ligand that interfaces at least two molecules, normally metal particles. The ligand might be nuclear or polyatomic. Essentially all intricate natural mixes can fill in as bridging ligands, so the term is normally limited to little ligands, for example, pseudo-halides or to ligands that are explicitly intended to connect two metals.
Note: In naming a complex wherein a solitary particle spans two metals, the connecting ligand is gone before by the Greek letter mu, $\mu $, with a superscript number indicating the quantity of metals bound to the crossing over the ligand. ${\mu _2}$ is frequently signified basically as $\mu $. While depicting coordination buildings care ought to be taken not to mistake $\mu $ for $\eta $, which identifies with hapticity. Ligands that are not connecting are called terminal ligands.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




