
The number of covalent bonds present in ${\text{Ca}}{{\text{C}}_{\text{2}}}$ is/are:
(A) ${\text{4}}$
(B) ${\text{3}}$
(C) ${\text{2}}$
(D) ${\text{1}}$
Answer
573.6k+ views
Hint: In a compound we have many types of bonds so we will draw structure first so that we can predict the type of bonding between them. When we predict the type of bonding between them then we can know the number of bonds as well.we also know that covalent bond formed due to electron sharing between 2 atoms
Complete step by step answer:
First we see the structure of the molecule
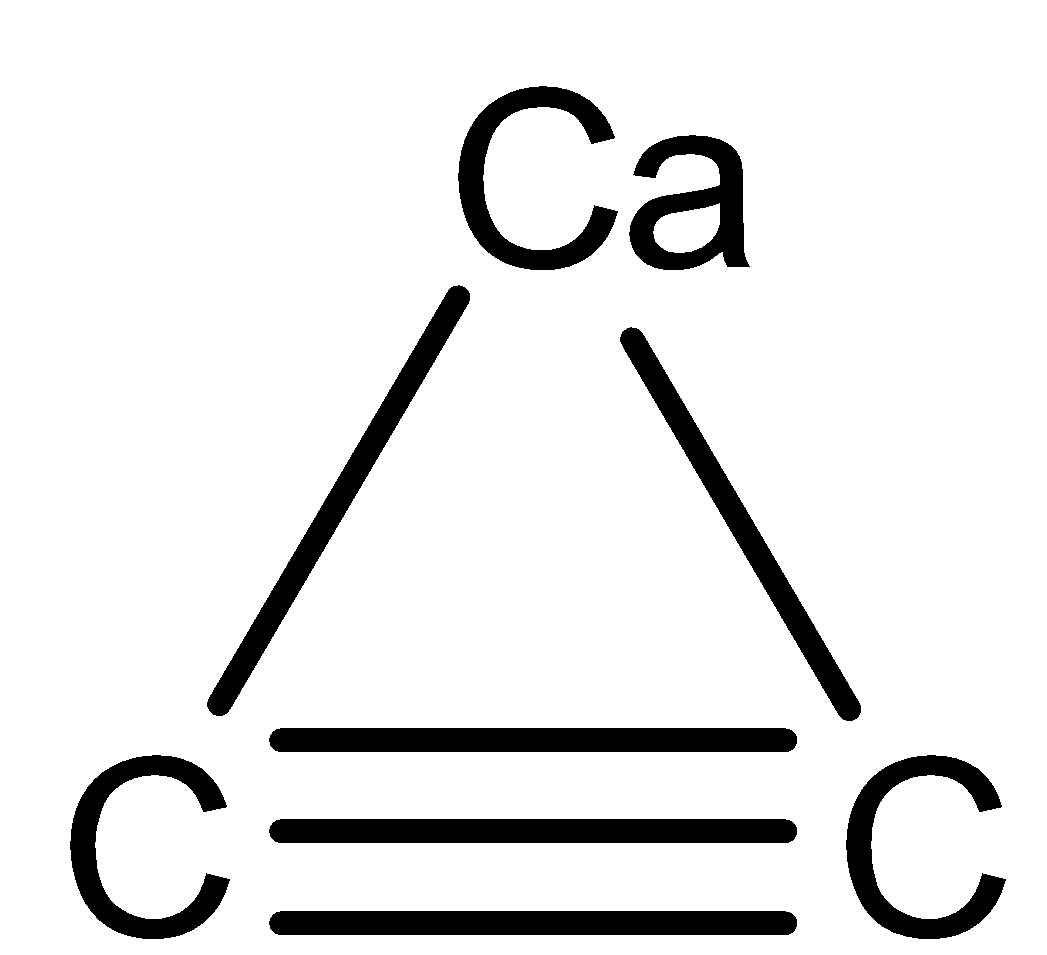
when we made ${\text{Ca}}{{\text{C}}_{\text{2}}}$ dissociate we see the ions which will tell us the number of ionic bonds present
${\text{Ca}}{{\text{C}}_{\text{2}}}{\text{ }} \to {\text{ C}}{{\text{a}}^{{\text{2 + }}}}{\text{ + C}}_{\text{2}}^{{\text{2 - }}}$
Now ${\text{Ca}}$don’t have any covalent bond between them or the carbon so now we will move on to ${\text{C}}_{\text{2}}^{{\text{2 - }}}$ as to how many bonds it have.
Now when we study double bond or triple bond, we study about $\sigma$ and $\pi$ bonds
In a double bond we have 1$\sigma$ and 2 $\pi$ present between two carbons.
In a triple bond we have 1$\sigma$ and 2 $\pi$ present between two carbons.
The 1$\sigma$ and 2 $\pi$ bonds both are covalent bonds. So, on seeing the structure of carbon carbide above we can see there are two types of bonds present in it. One is an ionic bond and other is a covalent bond.
In covalent we have 1$\sigma$ and 2 $\pi$ which sums upto \[3\] covalent bonds.
So, the correct answer is Option B.
Note: The formation of $\sigma$ bond is the result of the head-on overlapping of the overlapping while the formation of $\pi$ is the result of the lateral overlapping of the orbitals which are perpendicular to the head-on overlapping.
Ionic bond is present between ions which have charge on them while covalent bond is sharing bond in which there is no charge involved and between non-charged moieties.
Complete step by step answer:
First we see the structure of the molecule

when we made ${\text{Ca}}{{\text{C}}_{\text{2}}}$ dissociate we see the ions which will tell us the number of ionic bonds present
${\text{Ca}}{{\text{C}}_{\text{2}}}{\text{ }} \to {\text{ C}}{{\text{a}}^{{\text{2 + }}}}{\text{ + C}}_{\text{2}}^{{\text{2 - }}}$
Now ${\text{Ca}}$don’t have any covalent bond between them or the carbon so now we will move on to ${\text{C}}_{\text{2}}^{{\text{2 - }}}$ as to how many bonds it have.
Now when we study double bond or triple bond, we study about $\sigma$ and $\pi$ bonds
In a double bond we have 1$\sigma$ and 2 $\pi$ present between two carbons.
In a triple bond we have 1$\sigma$ and 2 $\pi$ present between two carbons.
The 1$\sigma$ and 2 $\pi$ bonds both are covalent bonds. So, on seeing the structure of carbon carbide above we can see there are two types of bonds present in it. One is an ionic bond and other is a covalent bond.
In covalent we have 1$\sigma$ and 2 $\pi$ which sums upto \[3\] covalent bonds.
So, the correct answer is Option B.
Note: The formation of $\sigma$ bond is the result of the head-on overlapping of the overlapping while the formation of $\pi$ is the result of the lateral overlapping of the orbitals which are perpendicular to the head-on overlapping.
Ionic bond is present between ions which have charge on them while covalent bond is sharing bond in which there is no charge involved and between non-charged moieties.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




