
The number of open chain isomers possible for ${C_4}{H_6}$:
A.$6$
B.$5$
C.$4$
D.$2$
Answer
522.5k+ views
Hint:In this question we will find the total number of open chain isomers possible for ${C_4}{H_6}$. We will use the basic concepts of isomerism and we will try to find open chain isomers only. We will exclude other isomers formed.
Complete step-by-step answer:First, we will understand the basic terms like ‘Isomers’ and ‘Open chain isomers’. So let’s understand these terms to find the exact number of open chain isomers possible for ${C_4}{H_6}$.
Isomers are molecules with the identical molecular formulas which means the same number of atoms of each element but the molecule will differ in the position of atoms and bonds. Now one more term ‘Open chain isomer’. It is one of the types of isomer in which a compound is acyclic with a linear structure rather than a cyclic one. An open chain isomer has no side chains.
Now we understand the basic terms used in the question. So we will find open chain isomers for ${C_4}{H_6}$.
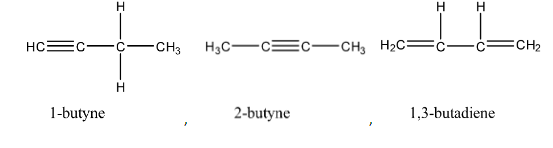
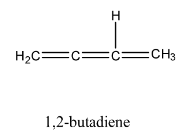
We can observe that the above molecules have identical molecular formula ${C_4}{H_6}$. Same number of carbon and hydrogen atoms but distinct arrangement of atoms and bonds.
In the question we are asked to find the total number of open chain isomers possible for ${C_4}{H_6}$. All above isomers are open chain because they are straight chain compounds, acyclic and isomers with no side chains.
We can observe that the total number of open chain isomers possible for ${C_4}{H_6}$ is $4$.
Therefore, the correct option is (C).
Note:The total number of isomers possible for ${C_4}{H_6}$ is $9$. Out of the $9$ isomers $4$ are open chain isomers and rest of them are cyclic isomers.
Isomers have identical molecular formula but their chemical and physical properties may not be the same.
Complete step-by-step answer:First, we will understand the basic terms like ‘Isomers’ and ‘Open chain isomers’. So let’s understand these terms to find the exact number of open chain isomers possible for ${C_4}{H_6}$.
Isomers are molecules with the identical molecular formulas which means the same number of atoms of each element but the molecule will differ in the position of atoms and bonds. Now one more term ‘Open chain isomer’. It is one of the types of isomer in which a compound is acyclic with a linear structure rather than a cyclic one. An open chain isomer has no side chains.
Now we understand the basic terms used in the question. So we will find open chain isomers for ${C_4}{H_6}$.


We can observe that the above molecules have identical molecular formula ${C_4}{H_6}$. Same number of carbon and hydrogen atoms but distinct arrangement of atoms and bonds.
In the question we are asked to find the total number of open chain isomers possible for ${C_4}{H_6}$. All above isomers are open chain because they are straight chain compounds, acyclic and isomers with no side chains.
We can observe that the total number of open chain isomers possible for ${C_4}{H_6}$ is $4$.
Therefore, the correct option is (C).
Note:The total number of isomers possible for ${C_4}{H_6}$ is $9$. Out of the $9$ isomers $4$ are open chain isomers and rest of them are cyclic isomers.
Isomers have identical molecular formula but their chemical and physical properties may not be the same.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




