
The number of possible isomers for ${{C}_{7}}{{H}_{8}}O$ retaining the phenyl ring in all of its isomers is as follows:
A.3
B.4
C.5
D.7
Answer
587.7k+ views
Hint: Isomers are molecules or polyatomic ions with identical molecular formulae, i.e., the same number of atoms of each element, but distinct arrangement of atoms in space. Isomerism is the existence or possibility of isomers.
Complete answer:
Isomers do not usually have identical chemical or physical properties. Two major types of isomerism are structural or constitutional isomerism, in which the bonds between the atoms vary; and stereoisomerism or spatial isomerism, in which the bonds are the same but the relative positions of atoms vary.
Given compound is ${{C}_{7}}{{H}_{8}}O$.
The possible number of isomers for the given compound ${{C}_{7}}{{H}_{8}}O$ are 5.
Naming the 5 possible isomers, they are benzyl alcohol, anisole, o-, m-, and p- cresols (methyl phenols). Defining all of the five isomers with their structure below:
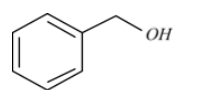
Benzyl alcohol is an aromatic alcohol of the formula ${{C}_{6}}{{H}_{5}}C{{H}_{2}}OH$. Benzyl alcohol is a colourless liquid with a moderate, pleasant aromatic smell.
Benzyl alcohol
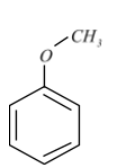
Anisole, or methoxybenzene, is an organic compound containing the formula $C{{H}_{3}}O{{C}_{6}}{{H}_{5}}$. It is a colourless liquid with an odour reminiscent of anise seed, and many of its derivatives are used in natural and artificial fragrances. It's the ether.
Anisole
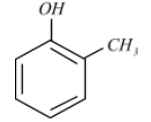
OrthoCresol, also 2methylphenol, is an organic compound containing the formula $C{{H}_{3}}{{C}_{6}}{{H}_{4}}\left( OH \right)$. It is a colourless solid that is widely used as an intermediate in the production of other chemicals.
o-Cresol
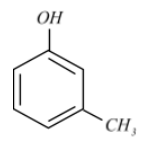
MetaCresol, also 3methylphenol, is an organic compound containing the formula $C{{H}_{3}}{{C}_{6}}{{H}_{4}}\left( OH \right)$. It is a colourless, viscous liquid that is used as an intermediate in the manufacture of other chemicals.
m-Cresol
Para Cresol, also 4methylphenol, is a chemical compound containing the formula $C{{H}_{3}}{{C}_{6}}{{H}_{4}}\left( OH \right)$.It is a colourless solid that is commonly used as an intermediate in the manufacture of other chemicals.
p-Cresol

So,the option C is the correct answer.
Note:
There are three kinds of structural isomers: chain isomers, functional group isomers, and positional isomers. Chain isomers have the same molecular formula but different structures or branches. Functional group isomers have the same formula, but they have different functional groups.
Complete answer:
Isomers do not usually have identical chemical or physical properties. Two major types of isomerism are structural or constitutional isomerism, in which the bonds between the atoms vary; and stereoisomerism or spatial isomerism, in which the bonds are the same but the relative positions of atoms vary.
Given compound is ${{C}_{7}}{{H}_{8}}O$.
The possible number of isomers for the given compound ${{C}_{7}}{{H}_{8}}O$ are 5.
Naming the 5 possible isomers, they are benzyl alcohol, anisole, o-, m-, and p- cresols (methyl phenols). Defining all of the five isomers with their structure below:
Benzyl alcohol is an aromatic alcohol of the formula ${{C}_{6}}{{H}_{5}}C{{H}_{2}}OH$. Benzyl alcohol is a colourless liquid with a moderate, pleasant aromatic smell.
Benzyl alcohol

Anisole, or methoxybenzene, is an organic compound containing the formula $C{{H}_{3}}O{{C}_{6}}{{H}_{5}}$. It is a colourless liquid with an odour reminiscent of anise seed, and many of its derivatives are used in natural and artificial fragrances. It's the ether.
Anisole

OrthoCresol, also 2methylphenol, is an organic compound containing the formula $C{{H}_{3}}{{C}_{6}}{{H}_{4}}\left( OH \right)$. It is a colourless solid that is widely used as an intermediate in the production of other chemicals.
o-Cresol

MetaCresol, also 3methylphenol, is an organic compound containing the formula $C{{H}_{3}}{{C}_{6}}{{H}_{4}}\left( OH \right)$. It is a colourless, viscous liquid that is used as an intermediate in the manufacture of other chemicals.
m-Cresol

Para Cresol, also 4methylphenol, is a chemical compound containing the formula $C{{H}_{3}}{{C}_{6}}{{H}_{4}}\left( OH \right)$.It is a colourless solid that is commonly used as an intermediate in the manufacture of other chemicals.
p-Cresol

So,the option C is the correct answer.
Note:
There are three kinds of structural isomers: chain isomers, functional group isomers, and positional isomers. Chain isomers have the same molecular formula but different structures or branches. Functional group isomers have the same formula, but they have different functional groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




