
The number of primary and secondary hydroxyl groups in ribose are:
Answer
570.3k+ views
Hint: First, we have to examine the structure of the ribose molecule and find out the positions of each hydroxyl group. Primary hydroxyl groups are those which are attached to primary carbon atoms, and secondary hydroxyl groups are those which are attached to secondary carbon atoms.
Complete step by step answer:
Let us first examine the structure of the ribose molecule:
It consists of five carbon atoms with an aldehyde group at one end, and several hydroxyl groups distributed as follows:
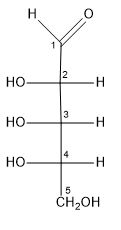
The numbers in the above image denote the respective carbon atoms.
The fifth carbon is a primary carbon atom, since it is bonded to only one other carbon atom. Hence, the hydroxyl group attached to it is a primary hydroxyl group. Therefore, the number of primary hydroxyl groups in ribose is one.
As we can see, the second, third and fourth carbon atoms are secondary carbon atoms, since each of them are further bonded to two other carbon atoms. Hence, the hydroxyl groups present on them are said to be secondary hydroxyl groups. Therefore, ribose has three hydroxyl groups.
Hence, from our observations, the number of primary and secondary hydroxyl groups in ribose are one and three respectively.
Note: Ribose is a simple pentose sugar that is an essential component of ribonucleotides which make up RNA of organisms. Like most sugars, it exists in a cyclic form as well, in addition to the linear structure shown in the image above. The type of structural projection shown in the image above is known as Fischer's projection. Note that ribose plays a major role in coding and translation of genes in organisms.
Complete step by step answer:
Let us first examine the structure of the ribose molecule:
It consists of five carbon atoms with an aldehyde group at one end, and several hydroxyl groups distributed as follows:

The numbers in the above image denote the respective carbon atoms.
The fifth carbon is a primary carbon atom, since it is bonded to only one other carbon atom. Hence, the hydroxyl group attached to it is a primary hydroxyl group. Therefore, the number of primary hydroxyl groups in ribose is one.
As we can see, the second, third and fourth carbon atoms are secondary carbon atoms, since each of them are further bonded to two other carbon atoms. Hence, the hydroxyl groups present on them are said to be secondary hydroxyl groups. Therefore, ribose has three hydroxyl groups.
Hence, from our observations, the number of primary and secondary hydroxyl groups in ribose are one and three respectively.
Note: Ribose is a simple pentose sugar that is an essential component of ribonucleotides which make up RNA of organisms. Like most sugars, it exists in a cyclic form as well, in addition to the linear structure shown in the image above. The type of structural projection shown in the image above is known as Fischer's projection. Note that ribose plays a major role in coding and translation of genes in organisms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




