
The number of resonating structure of phenoxide ion is:
A. Four
B. five
C. six
D. seven
Answer
568.8k+ views
:Hint:Phenoxide ions contain non-equivalent resonance structures in which negative charge is there which is less effectively delocalised over less electronegative carbon atom and 1 oxygen atom.
Complete answer:
First we need to see the structure of Phenoxide ion or Phenolate ion. Phenoxide has the molecular formula ${{C_6}}{{H_5}}O$ and its molecular weight is 93.1 g/mol. Phenoxide ions contain non-equivalent resonance structures in which negative charge is there which is less effectively delocalised over less electronegative carbon atom and 1 oxygen atom.
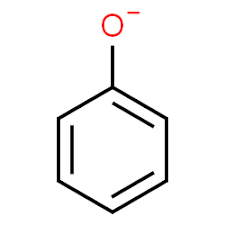
Phenoxide ion
Above shown figure is phenoxide ion, now we need to see the resonating structures
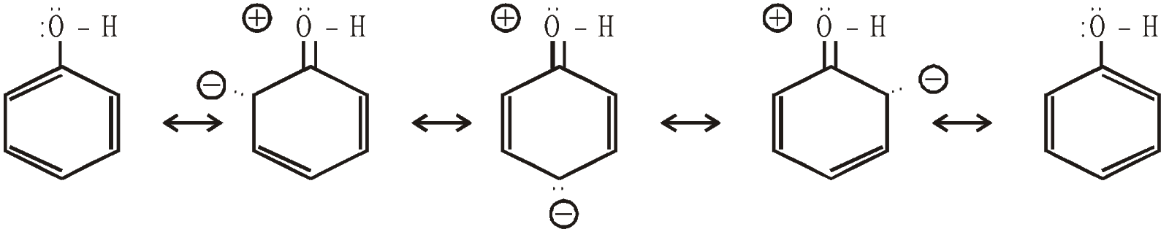
Above shown figure is about resonating structures of phenoxide ions and as we can see it has 5 resonating structures among which two structures are repeating as they are in starting and in ending too.
Hence, Option B is correct.
Additional Information:
Phenolate or phenoxide ion is an anion that is the conjugate base of phenol formed by deprotonation of the OH group. It has a big role as a human xenobiotic metabolite. Actually it is a conjugate base of a phenol.
Note:
Resonance structures can be defined as sets of Lewis structures which best describe the delocalization of electrons in a polyatomic ion or in a molecule. In many of the cases, a single Lewis structure is not enough to express the bonding in a molecule/polyatomic ion because of the existence of partial charges and fractional bonds in it.Firstly, It is very important to draw the figure of Phenoxide ion and then we should try in creating the resonating structures of phenoxide ion.
Complete answer:
First we need to see the structure of Phenoxide ion or Phenolate ion. Phenoxide has the molecular formula ${{C_6}}{{H_5}}O$ and its molecular weight is 93.1 g/mol. Phenoxide ions contain non-equivalent resonance structures in which negative charge is there which is less effectively delocalised over less electronegative carbon atom and 1 oxygen atom.

Phenoxide ion
Above shown figure is phenoxide ion, now we need to see the resonating structures

Above shown figure is about resonating structures of phenoxide ions and as we can see it has 5 resonating structures among which two structures are repeating as they are in starting and in ending too.
Hence, Option B is correct.
Additional Information:
Phenolate or phenoxide ion is an anion that is the conjugate base of phenol formed by deprotonation of the OH group. It has a big role as a human xenobiotic metabolite. Actually it is a conjugate base of a phenol.
Note:
Resonance structures can be defined as sets of Lewis structures which best describe the delocalization of electrons in a polyatomic ion or in a molecule. In many of the cases, a single Lewis structure is not enough to express the bonding in a molecule/polyatomic ion because of the existence of partial charges and fractional bonds in it.Firstly, It is very important to draw the figure of Phenoxide ion and then we should try in creating the resonating structures of phenoxide ion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




