
The number of sigma and pi bonds in benzene are:
a. 6 sigma and 3 pi bonds
b. 12 sigma and 3 pi bonds
c. 9 sigma and 3 pi bonds
c. 6 sigma and 6 pi bonds
Answer
597.3k+ views
Hint: Benzene is a hydrocarbon composed of six carbons connected via covalent bond, and exists in the form of a ring. It has three alternating double bonds which makes the compound very stable.
Complete step by step answer:
Benzene is an aromatic compound of carbon and hydrogen. The molecular formula for benzene is \[{{C}_{6}}{{H}_{6}}\].
Let us draw the structure of Benzene.
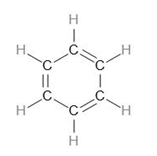
To calculate the number of sigma bonds in this, we can draw the skeletal structure by only drawing the sigma bonds –
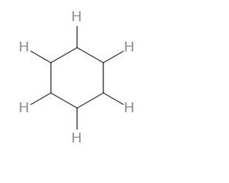
As we can see, there are 6 C-H bonds, 6 C-C bonds.
Therefore, we can say that there is a total of 12 sigma bonds in benzene.
Now, looking at the structure of benzene, we can see that there are 3 C=C bonds.
Therefore, there are 12 sigma bonds and 3 pi bonds. Benzene is therefore made up of 15 covalent bonds.
Therefore, the answer is – option (b) – The number of sigma and pi bonds in benzene are 12 and 3, respectively.
Additional Information: One single bond contains one sigma bond; a double bond contains one sigma and one pi bond.Similarly, a triple bond contains one sigma and two pi bonds.
Note: Benzene exists as a colorless and highly flammable liquid with a sweet smell. This sweet smell is produced as a result of aromaticity of the compound. Aromaticity of benzene arises due to the continuous cyclic pi bonds between the carbon atoms. It is a very stable compound. Benzene is naturally present in crude oil and is also an elementary petrochemical.
Complete step by step answer:
Benzene is an aromatic compound of carbon and hydrogen. The molecular formula for benzene is \[{{C}_{6}}{{H}_{6}}\].
Let us draw the structure of Benzene.

To calculate the number of sigma bonds in this, we can draw the skeletal structure by only drawing the sigma bonds –

As we can see, there are 6 C-H bonds, 6 C-C bonds.
Therefore, we can say that there is a total of 12 sigma bonds in benzene.
Now, looking at the structure of benzene, we can see that there are 3 C=C bonds.
Therefore, there are 12 sigma bonds and 3 pi bonds. Benzene is therefore made up of 15 covalent bonds.
Therefore, the answer is – option (b) – The number of sigma and pi bonds in benzene are 12 and 3, respectively.
Additional Information: One single bond contains one sigma bond; a double bond contains one sigma and one pi bond.Similarly, a triple bond contains one sigma and two pi bonds.
Note: Benzene exists as a colorless and highly flammable liquid with a sweet smell. This sweet smell is produced as a result of aromaticity of the compound. Aromaticity of benzene arises due to the continuous cyclic pi bonds between the carbon atoms. It is a very stable compound. Benzene is naturally present in crude oil and is also an elementary petrochemical.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




