
The number of S-S bond in sulphur trioxide trimer is:
A. 3
B. 2
C. 1
D. 0
Answer
565.5k+ views
Hint: As we know that sulphur trioxide trimer has the chemical formula $\left( {{S}_{3}}{{O}_{9}} \right)$. We can say it has nine oxygen atoms and three sulphur atoms. It is found that the chemical formula of sulphur trioxide is $S{{O}_{3}}$
Complete answer:
- As we know that sulphur trioxide is a colourless liquid, that can exist as fibre- like crystals or as a gas. It is found that when sulphur trioxide is exposed to air, it takes the water molecules and in turn releases white fumes.
- First of all let’s draw the structure of sulphur trioxide trimer $\left( {{S}_{3}}{{O}_{9}} \right)$-
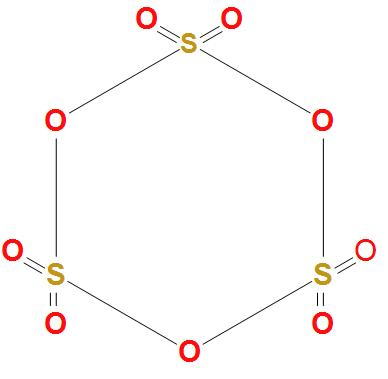
- We can see in the above structure of sulphur trioxide trimer $\left( {{S}_{3}}{{O}_{9}} \right)$ that; all the three sulphur atoms are bonded to the oxygen atoms on all the sides. It is clear that there is no bond formation taking place in between the sulphur atoms.
- It is found that sulphur trioxide is also called sulphur oxide, it is used for the production of sulphuric acid and for other explosives as well as for chemicals.
Hence, we can conclude that the correct option is (d), that is the number of S-S bond in sulphur trioxide trimer $\left( {{S}_{3}}{{O}_{9}} \right)$ is 0.
Note:
- We should note here that there exist no bonds between the sulphur atoms in sulphur trioxide trimer. There are basically six S-O bonds and three S-O-S bonds in sulphur trioxide trimer compounds.
Complete answer:
- As we know that sulphur trioxide is a colourless liquid, that can exist as fibre- like crystals or as a gas. It is found that when sulphur trioxide is exposed to air, it takes the water molecules and in turn releases white fumes.
- First of all let’s draw the structure of sulphur trioxide trimer $\left( {{S}_{3}}{{O}_{9}} \right)$-

- We can see in the above structure of sulphur trioxide trimer $\left( {{S}_{3}}{{O}_{9}} \right)$ that; all the three sulphur atoms are bonded to the oxygen atoms on all the sides. It is clear that there is no bond formation taking place in between the sulphur atoms.
- It is found that sulphur trioxide is also called sulphur oxide, it is used for the production of sulphuric acid and for other explosives as well as for chemicals.
Hence, we can conclude that the correct option is (d), that is the number of S-S bond in sulphur trioxide trimer $\left( {{S}_{3}}{{O}_{9}} \right)$ is 0.
Note:
- We should note here that there exist no bonds between the sulphur atoms in sulphur trioxide trimer. There are basically six S-O bonds and three S-O-S bonds in sulphur trioxide trimer compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




