
The numbers of lone pair(s) on ${\text{Xe}}$ in ${\text{Xe}}{{\text{F}}_{\text{2}}}$ and ${\text{Xe}}{{\text{F}}_4}$ are, respectively:
A) 2 and 3
B) 4 and 1
C) 3 and 2
D) 4 and 2
Answer
585k+ views
Hint: Using the periodic table calculate the total number of valence electrons of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ and ${\text{Xe}}{{\text{F}}_4}$. Draw the Lewis dot structures of both the compounds and determine the number of lone pair on ${\text{Xe}}$ in ${\text{Xe}}{{\text{F}}_{\text{2}}}$ and ${\text{Xe}}{{\text{F}}_4}$.
Formula Used: Number of valence electrons of an element = Group number of an element
Number of valence electrons of an element = Sum of the valence electrons of all atoms
Complete step by step answer:
Calculate the total number of valence electrons of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ and ${\text{Xe}}{{\text{F}}_4}$.
Number of valence electrons of an element = Group number of an element
An element Xe is a Noble gas element and present in group number 8 of the periodic table. While F is halogen and present in group number 7 of the periodic table.
Number of valence electrons of Xe = 8
Number of valence electrons of F = 7
$ {\text{Total no}}{\text{. of valence electrons of Xe}}{{\text{F}}_{\text{2}}} = {\text{ (No}}{\text{. of Xe atoms) (No}}{\text{. of valence electrons of Xe) + (No}}{\text{. of F atoms) }} \\
{\text{ (No}}{\text{. of valence electrons of F)}} \\ $
${\text{Total no}}{\text{. of valence electrons of Xe}}{{\text{F}}_{\text{2}}} = {\text{ (1) (8) + (2)(7) = 22 electrons}}$
Similarly, we can calculate the total number of valence electrons of ${\text{Xe}}{{\text{F}}_4}$ as follows:
${\text{Total no}}{\text{. of valence electrons of Xe}}{{\text{F}}_4} = {\text{ (1) (8) + (4)(7) = 36 electrons}}$
Using the total number of valence electrons draw the Lewis dot structures of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ and ${\text{Xe}}{{\text{F}}_4}$ as follows:
A less electronegative atom is always a central atom except for ${\text{H}}$ atom.
So, in both the compounds the central atom is ${\text{Xe}}$.
Skeleton structures of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ and ${\text{Xe}}{{\text{F}}_4}$ are
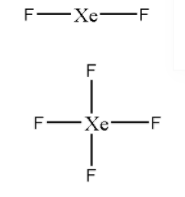
Now, let us distribute the valence electrons to complete the octet of surrounding atoms.
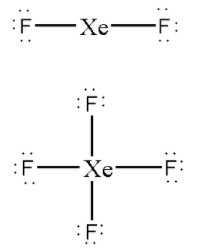
Now, let us distribute the remaining valence electrons around the central atom.
Out of total 22 valence electrons of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ we have distributed 4 electrons in bonding and 12 electrons as lone pairs around ${\text{F}}$ atoms.
So, now distribute the remaining 6 electrons as 3 lone pairs on the central ${\text{Xe}}$ atom.
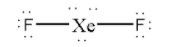
Thus, there are 3 lone pairs on the central ${\text{Xe}}$ atom in ${\text{Xe}}{{\text{F}}_{\text{2}}}$.
Out of total 36 valence electrons of ${\text{Xe}}{{\text{F}}_4}$ we have distributed 8 electrons in bonding and 24 electrons as lone pairs around ${\text{F}}$ atoms.
Now, distribute the remaining 4 electrons as 2 lone pairs on the central ${\text{Xe}}$ atom.
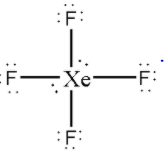
Thus, there are 2 lone pairs on the central ${\text{Xe}}$ atom in${\text{Xe}}{{\text{F}}_4}$.
Hence, the correct option is (C) 3 and 2.
Note: Here in both the compounds central ${\text{Xe}}$ atom show an exception to the octet rule. In the case of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ a central ${\text{Xe}}$ atom is surrounded by 10 electrons and in the case of ${\text{Xe}}{{\text{F}}_4}$ a central ${\text{Xe}}$ atom is surrounded by 12 electrons.
Formula Used: Number of valence electrons of an element = Group number of an element
Number of valence electrons of an element = Sum of the valence electrons of all atoms
Complete step by step answer:
Calculate the total number of valence electrons of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ and ${\text{Xe}}{{\text{F}}_4}$.
Number of valence electrons of an element = Group number of an element
An element Xe is a Noble gas element and present in group number 8 of the periodic table. While F is halogen and present in group number 7 of the periodic table.
Number of valence electrons of Xe = 8
Number of valence electrons of F = 7
$ {\text{Total no}}{\text{. of valence electrons of Xe}}{{\text{F}}_{\text{2}}} = {\text{ (No}}{\text{. of Xe atoms) (No}}{\text{. of valence electrons of Xe) + (No}}{\text{. of F atoms) }} \\
{\text{ (No}}{\text{. of valence electrons of F)}} \\ $
${\text{Total no}}{\text{. of valence electrons of Xe}}{{\text{F}}_{\text{2}}} = {\text{ (1) (8) + (2)(7) = 22 electrons}}$
Similarly, we can calculate the total number of valence electrons of ${\text{Xe}}{{\text{F}}_4}$ as follows:
${\text{Total no}}{\text{. of valence electrons of Xe}}{{\text{F}}_4} = {\text{ (1) (8) + (4)(7) = 36 electrons}}$
Using the total number of valence electrons draw the Lewis dot structures of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ and ${\text{Xe}}{{\text{F}}_4}$ as follows:
A less electronegative atom is always a central atom except for ${\text{H}}$ atom.
So, in both the compounds the central atom is ${\text{Xe}}$.
Skeleton structures of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ and ${\text{Xe}}{{\text{F}}_4}$ are

Now, let us distribute the valence electrons to complete the octet of surrounding atoms.

Now, let us distribute the remaining valence electrons around the central atom.
Out of total 22 valence electrons of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ we have distributed 4 electrons in bonding and 12 electrons as lone pairs around ${\text{F}}$ atoms.
So, now distribute the remaining 6 electrons as 3 lone pairs on the central ${\text{Xe}}$ atom.
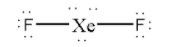
Thus, there are 3 lone pairs on the central ${\text{Xe}}$ atom in ${\text{Xe}}{{\text{F}}_{\text{2}}}$.
Out of total 36 valence electrons of ${\text{Xe}}{{\text{F}}_4}$ we have distributed 8 electrons in bonding and 24 electrons as lone pairs around ${\text{F}}$ atoms.
Now, distribute the remaining 4 electrons as 2 lone pairs on the central ${\text{Xe}}$ atom.

Thus, there are 2 lone pairs on the central ${\text{Xe}}$ atom in${\text{Xe}}{{\text{F}}_4}$.
Hence, the correct option is (C) 3 and 2.
Note: Here in both the compounds central ${\text{Xe}}$ atom show an exception to the octet rule. In the case of ${\text{Xe}}{{\text{F}}_{\text{2}}}$ a central ${\text{Xe}}$ atom is surrounded by 10 electrons and in the case of ${\text{Xe}}{{\text{F}}_4}$ a central ${\text{Xe}}$ atom is surrounded by 12 electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




