
The O−H bond length in \[{{\text{H}}_2}{\text{O}}\] is x $\mathop A\limits^ \circ $. The O−H bond length in \[{{\text{H}}_2}{{\text{O}}_2}\] is:
Answer
508.8k+ views
Hint: \[{{\text{H}}_2}{{\text{O}}_2}\] 's OH bond length is 98.8 pm in the solid state and 95 pm in the gaseous state. In water, however, the OH bond length is still 96pm.
The most basic form of peroxide is hydrogen peroxide (oxygen-oxygen single bond). It's a colourless substance that's used in aqueous solutions to keep things clean. It is used as a disinfectant as well as a bleaching agent. In rocketry, concentrated hydrogen peroxide is used as a propellant because it is a highly reactive oxygen species.
Complete answer:
The most basic form of peroxide is hydrogen peroxide (oxygen-oxygen single bond). It's a colourless substance that's used in aqueous solutions to keep things clean. It is used as a disinfectant as well as a bleaching agent. In rocketry, concentrated hydrogen peroxide is used as a propellant because it is a highly reactive oxygen species.
The distance between the nuclei of two chemically bound atoms in a molecule is measured by bond length. It is roughly proportional to the number of the two bonded atoms' covalent radii. Bond length is inversely proportional to bond order in covalent bonds; higher bond orders result in stronger bonds, which are followed by stronger forces of attraction that tie the atoms together. These powerful powers of attraction result in short bonds.
Hydrogen peroxide has a non-planar composition. With O – O flips, \[{{\text{H}}_2}{{\text{O}}_2}\] has an open book structure. 111° is the dihedral angle. The O-O bond is 145.8 pm long, while the O-H bond is 98.8 pm long. Its arrangement is divided into two planes, each with one O-H bond pair; the angle between the two planes is 90.2°.
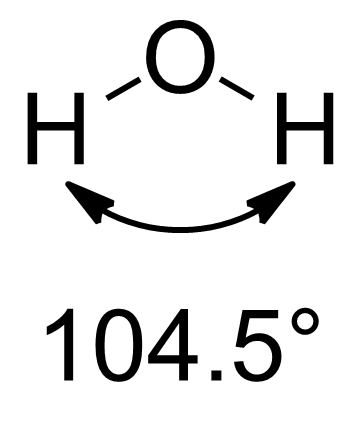
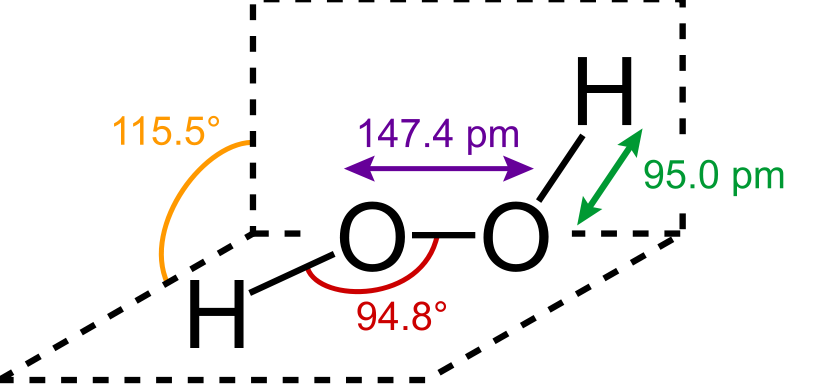
\[{{\text{H}}_2}{{\text{O}}_2}\] 's OH bond length is 98.8 pm in the solid state and 95 pm in the gaseous state. In water, however, the OH bond length is still 96pm.
Hence Option A < x $\mathop A\limits^ \circ $ is correct.
Note:
Hydrogen peroxide has a non-planar composition. With O – O flips, \[{{\text{H}}_2}{{\text{O}}_2}\] has an open book structure. 111° is the dihedral angle. The O-O bond is 145.8 pm long, while the O-H bond is 98.8 pm long. Its arrangement is divided into two planes, each with one O-H bond pair; the angle between the two planes is 90.2°.
The most basic form of peroxide is hydrogen peroxide (oxygen-oxygen single bond). It's a colourless substance that's used in aqueous solutions to keep things clean. It is used as a disinfectant as well as a bleaching agent. In rocketry, concentrated hydrogen peroxide is used as a propellant because it is a highly reactive oxygen species.
Complete answer:
The most basic form of peroxide is hydrogen peroxide (oxygen-oxygen single bond). It's a colourless substance that's used in aqueous solutions to keep things clean. It is used as a disinfectant as well as a bleaching agent. In rocketry, concentrated hydrogen peroxide is used as a propellant because it is a highly reactive oxygen species.
The distance between the nuclei of two chemically bound atoms in a molecule is measured by bond length. It is roughly proportional to the number of the two bonded atoms' covalent radii. Bond length is inversely proportional to bond order in covalent bonds; higher bond orders result in stronger bonds, which are followed by stronger forces of attraction that tie the atoms together. These powerful powers of attraction result in short bonds.
Hydrogen peroxide has a non-planar composition. With O – O flips, \[{{\text{H}}_2}{{\text{O}}_2}\] has an open book structure. 111° is the dihedral angle. The O-O bond is 145.8 pm long, while the O-H bond is 98.8 pm long. Its arrangement is divided into two planes, each with one O-H bond pair; the angle between the two planes is 90.2°.


\[{{\text{H}}_2}{{\text{O}}_2}\] 's OH bond length is 98.8 pm in the solid state and 95 pm in the gaseous state. In water, however, the OH bond length is still 96pm.
Hence Option A < x $\mathop A\limits^ \circ $ is correct.
Note:
Hydrogen peroxide has a non-planar composition. With O – O flips, \[{{\text{H}}_2}{{\text{O}}_2}\] has an open book structure. 111° is the dihedral angle. The O-O bond is 145.8 pm long, while the O-H bond is 98.8 pm long. Its arrangement is divided into two planes, each with one O-H bond pair; the angle between the two planes is 90.2°.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




