
The oil of wintergreen is:
A.Ethyl salicylate
B.Methyl salicylate
C.Benzaldehyde
D.Phenyl salicylate
Answer
582k+ views
Hint: Oil of wintergreen or wintergreen oil is an organic compound with the formula ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}\left( {{\text{OH}}} \right)\left( {{\text{C}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}} \right)$ . The oil of wintergreen is an ester of salicylic acid and methanol.
Complete step by step answer:
The structure of the oil of wintergreen or wintergreen oil is shown below.
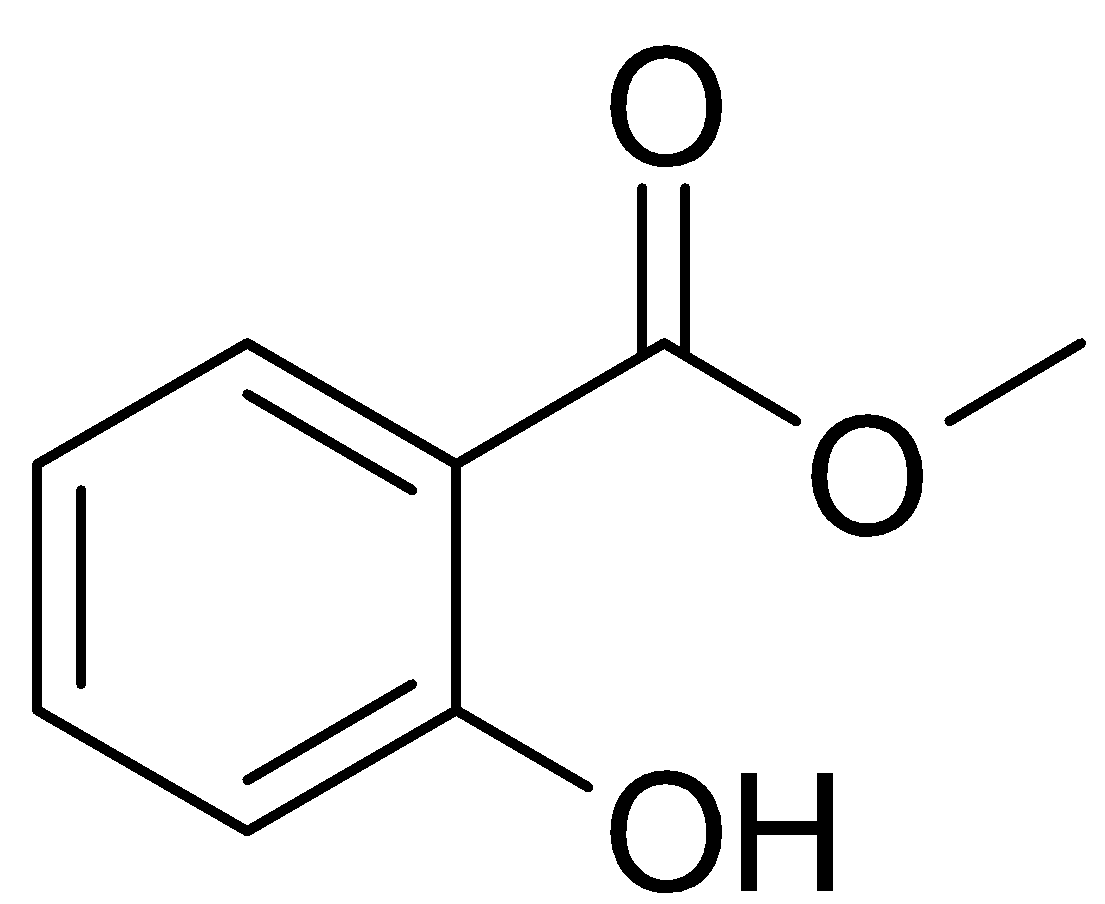
From the structure, it can be understood that the oil of wintergreen is a methyl ester of salicylic acid.
The oil of wintergreen is a colorless and viscous liquid. It has a sweet and fruity odor which bears a resemblance to root beer. It is often used in mint candies and hence also associatively named as ‘minty’.
The oil of wintergreen is produced by many types of plant species, but most specially it is produced by the wintergreen plants and hence the name.
The methyl salicylate or oil of wintergreen was first isolated from the plant American wintergreen by the French chemist Auguste Andre Thomas Cahours.
The biosynthesis of methyl salicylate or the oil of wintergreen takes place through the process of hydroxylation of benzoic acid by a cytochrome P – 450. This is followed by the methylation by a methylase enzyme.
Commercially, methyl salicylate is produced by the esterification of salicylic acid with methanol.
The production of methyl salicylate by plants is probably as an anti-herbivore defense because if the plant gets infected with herbivorous insects, then the release of this methyl salicylate helps the beneficial insects in killing the herbivorous insects.
Thus, oil of wintergreen is methyl salicylate.
Hence the correct option is B.
Note:
Methyl salicylate in high concentrations is used as an analgesic to treat joint and muscular pain.
In low concentrations, it is used as a flavouring agent in chewing gums and mints.
It is also used as an antiseptic in some mouthwashes.
Complete step by step answer:
The structure of the oil of wintergreen or wintergreen oil is shown below.

From the structure, it can be understood that the oil of wintergreen is a methyl ester of salicylic acid.
The oil of wintergreen is a colorless and viscous liquid. It has a sweet and fruity odor which bears a resemblance to root beer. It is often used in mint candies and hence also associatively named as ‘minty’.
The oil of wintergreen is produced by many types of plant species, but most specially it is produced by the wintergreen plants and hence the name.
The methyl salicylate or oil of wintergreen was first isolated from the plant American wintergreen by the French chemist Auguste Andre Thomas Cahours.
The biosynthesis of methyl salicylate or the oil of wintergreen takes place through the process of hydroxylation of benzoic acid by a cytochrome P – 450. This is followed by the methylation by a methylase enzyme.
Commercially, methyl salicylate is produced by the esterification of salicylic acid with methanol.
The production of methyl salicylate by plants is probably as an anti-herbivore defense because if the plant gets infected with herbivorous insects, then the release of this methyl salicylate helps the beneficial insects in killing the herbivorous insects.
Thus, oil of wintergreen is methyl salicylate.
Hence the correct option is B.
Note:
Methyl salicylate in high concentrations is used as an analgesic to treat joint and muscular pain.
In low concentrations, it is used as a flavouring agent in chewing gums and mints.
It is also used as an antiseptic in some mouthwashes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




