
The orbital picture of a triplet carbene can be drawn as :
A.
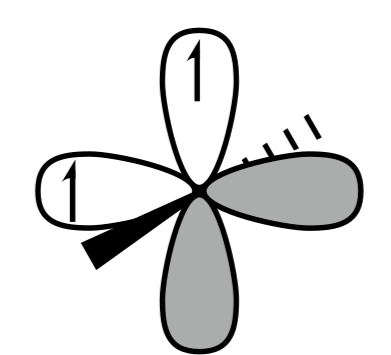
B.
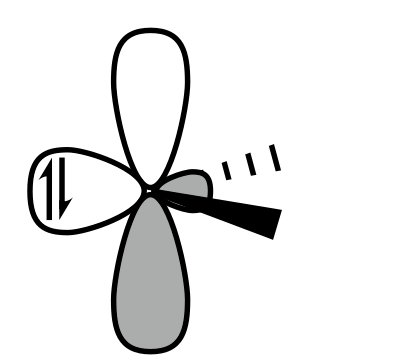
C.
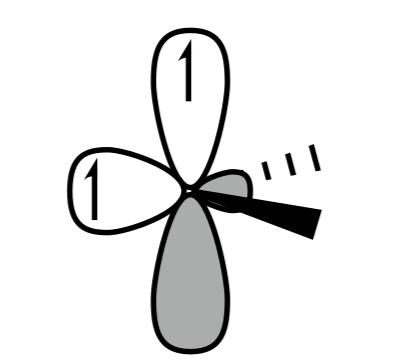
D. None of these



Answer
537.6k+ views
Hint :Triplet carbenes have two unpaired electrons. Bond angles are 125° to 140° for triplet methylene and 102° for singlet methylene. Generally stable in the gaseous state, while singlet carbenes occur more often in aqueous media.
Complete Step By Step Answer:
Carbene is a neutral, electron deficient species, there are two types of carbene singlet and triplet. Triplet carbenes have two unpaired electrons. Bond angles are 125-140° for triplet methylene and 102° for singlet methylene as determined by EPR.
Triplet carbenes are generally stable in the gaseous state, while singlet carbenes occur more often in aqueous media.
Triplet carbene is more stable than singlet carbene because it has two unpaired electrons and it has 33kJ/mol energy i.e lower than singlet carbene and triplet carbene is present in ground state which is more stable than excited state but singlet carbene is present in excited state
Singlet carbene is less stable than triplet.
Singlet carbene is shown in IMAGE B.
Triplet carbene is shown in IMAGE A and C
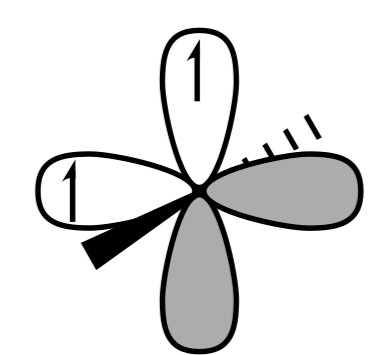
A.
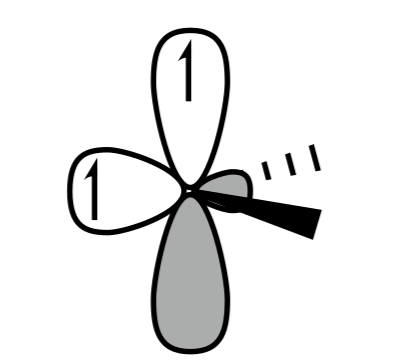
C.
Hence option A and C are the correct answer.
Note :
In the ground state, a singlet carbene has a pair of electrons in a single orbital, whereas the triplet has two unpaired electrons, each occupying a separate orbital. The designations singlet and triplet originate in spectroscopy.
Complete Step By Step Answer:
Carbene is a neutral, electron deficient species, there are two types of carbene singlet and triplet. Triplet carbenes have two unpaired electrons. Bond angles are 125-140° for triplet methylene and 102° for singlet methylene as determined by EPR.
Triplet carbenes are generally stable in the gaseous state, while singlet carbenes occur more often in aqueous media.
Triplet carbene is more stable than singlet carbene because it has two unpaired electrons and it has 33kJ/mol energy i.e lower than singlet carbene and triplet carbene is present in ground state which is more stable than excited state but singlet carbene is present in excited state
Singlet carbene is less stable than triplet.
Singlet carbene is shown in IMAGE B.
Triplet carbene is shown in IMAGE A and C

A.

C.
Hence option A and C are the correct answer.
Note :
In the ground state, a singlet carbene has a pair of electrons in a single orbital, whereas the triplet has two unpaired electrons, each occupying a separate orbital. The designations singlet and triplet originate in spectroscopy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




