
The order of stability of the following tautomeric compounds is:
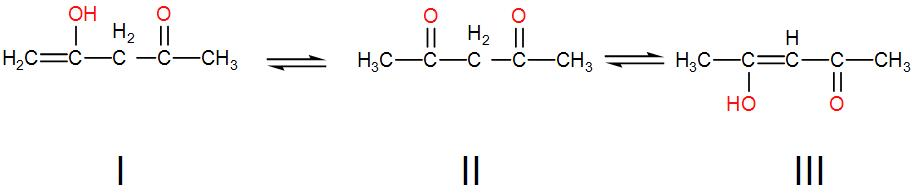
[A] I > II > III
[B] III > II> I
[C] II > I > III
[D] II > III > I

Answer
583.2k+ views
Hint: Tautomerism is a kind of isomerism. In this, there is an interconversion between the two possible chemical forms of the same species. There are sometimes tautomers which are more stable than the other one but an equilibrium is maintained between the forms.
Complete step by step answer:
We can define tautomers as the structural isomers of chemical compounds which they readily interconvert into each other. Generally, it’s the proton that relocates and thus there exists equilibrium between the two forms.
We call this process of interconverting of protons as tautomerization and the species undergoing the process are called the tautomers.
Here in the given compound we have keto – enol tautomerism. In this tautomerism, a compounds containing ketone groups i.e. –CO can undergo enol formation i.e. –CO converts to –COH even though Keto forms are considered more stable.
Now, let us see the structures given to us one by one.
In the third tautomer we have conjugated double bonds which give extra stability so III is the most stable.
Among the first and second tautomers, the first tautomer is the enol form and the second is the keto form. We know that keto is more stable than enol tautomer so structure II is more stable than structure I.
We can understand from the above discussion that the order of stability is III > II > I.
So, the correct answer is “Option B”.
Note: We should remember that tautomers are not similar to the canonical forms of resonance stabilised species. We can chemically distinguish tautomers and they can be identified by their different spectroscopic data. On the other hand resonance canonical structures do not physically exist and are just a hypothetical concept to understand theory of chemical compounds better.
Complete step by step answer:
We can define tautomers as the structural isomers of chemical compounds which they readily interconvert into each other. Generally, it’s the proton that relocates and thus there exists equilibrium between the two forms.
We call this process of interconverting of protons as tautomerization and the species undergoing the process are called the tautomers.
Here in the given compound we have keto – enol tautomerism. In this tautomerism, a compounds containing ketone groups i.e. –CO can undergo enol formation i.e. –CO converts to –COH even though Keto forms are considered more stable.
Now, let us see the structures given to us one by one.
In the third tautomer we have conjugated double bonds which give extra stability so III is the most stable.
Among the first and second tautomers, the first tautomer is the enol form and the second is the keto form. We know that keto is more stable than enol tautomer so structure II is more stable than structure I.
We can understand from the above discussion that the order of stability is III > II > I.
So, the correct answer is “Option B”.
Note: We should remember that tautomers are not similar to the canonical forms of resonance stabilised species. We can chemically distinguish tautomers and they can be identified by their different spectroscopic data. On the other hand resonance canonical structures do not physically exist and are just a hypothetical concept to understand theory of chemical compounds better.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




