
The ortho and para hydrogen differ in respect of which of the following?
(A) In the molecular weight
(B) In the nature of spin of protons
(C) In the nature of spin of electrons
(D) In the number of protons
Answer
588.9k+ views
Hint: Ortho and para hydrogen are the spin isomers of hydrogen where one of them has both nuclei rotating in the same direction while other has them rotating in different directions.
Complete step by step answer:
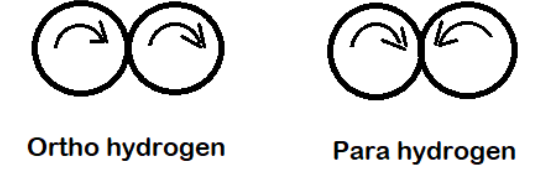
-Ortho and para hydrogen are the 2 spin isomers of hydrogen also known as allotropes of hydrogen.
-1 molecule of dihydrogen (${H_2}$) contains 2 atoms of hydrogen and the nuclei of both the hydrogen atoms is spinning. Based on the spin of the nuclei of the 2 hydrogen atoms the hydrogen are of 2 types namely: ortho and para hydrogen.
-The hydrogen molecule in which the spin of both the nuclei or the protons are in the same direction are known as ortho hydrogen. Since the spin is in one direction the net spin will be 1.
The hydrogen molecules in which the spin of both the nuclei or the protons are in opposite directions are known as para hydrogen. Since the spin is in 2 opposite directions the net spin will be 0.
So, we can see that the ortho and para hydrogen differ in the nuclear spin or the spin of protons.
-Para hydrogen is more stable than ortho hydrogen due to its 0 net spin. Para hydrogen also has a lower energy state than ortho hydrogen.
-The ordinary hydrogen we study about is an equilibrium mixture of both the ortho and para hydrogen molecules.
So, the correct option is: (B) In the nature of spin of protons.
Note:
The nucleus contains protons in it and so nuclear spin is known as proton spin. Also the concentration of ortho and para hydrogen varies according to temperature. At 0 K the hydrogen is mainly para and so is more stable. At room temperature the ortho and para hydrogen are present at a ratio of 3:1. Even at very high temperatures we can never obtain ortho to para ratio more than 3:1.
Complete step by step answer:
-Ortho and para hydrogen are the 2 spin isomers of hydrogen also known as allotropes of hydrogen.
-1 molecule of dihydrogen (${H_2}$) contains 2 atoms of hydrogen and the nuclei of both the hydrogen atoms is spinning. Based on the spin of the nuclei of the 2 hydrogen atoms the hydrogen are of 2 types namely: ortho and para hydrogen.
-The hydrogen molecule in which the spin of both the nuclei or the protons are in the same direction are known as ortho hydrogen. Since the spin is in one direction the net spin will be 1.
The hydrogen molecules in which the spin of both the nuclei or the protons are in opposite directions are known as para hydrogen. Since the spin is in 2 opposite directions the net spin will be 0.
So, we can see that the ortho and para hydrogen differ in the nuclear spin or the spin of protons.

-Para hydrogen is more stable than ortho hydrogen due to its 0 net spin. Para hydrogen also has a lower energy state than ortho hydrogen.
-The ordinary hydrogen we study about is an equilibrium mixture of both the ortho and para hydrogen molecules.
So, the correct option is: (B) In the nature of spin of protons.
Note:
The nucleus contains protons in it and so nuclear spin is known as proton spin. Also the concentration of ortho and para hydrogen varies according to temperature. At 0 K the hydrogen is mainly para and so is more stable. At room temperature the ortho and para hydrogen are present at a ratio of 3:1. Even at very high temperatures we can never obtain ortho to para ratio more than 3:1.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




