
The oxidation numbers of the sulphur atoms in peroxomonosulphuric acid ${{H}_{2}}S{{O}_{5}}$and peroxodisulphuric acid ${{H}_{2}}{{S}_{2}}{{O}_{8}}$are respectively.
A. +8and +7
B. +3 and +3
C. +6 and+6
D. +4 and +6
Answer
593.7k+ views
Hint:
To find the oxidation numbers we should know the charges present on each atom. Here, the compounds given are having peroxy linkage that is there is presence of a O-O single bond.
Complete step by step answer:
- Let’s first find the oxidation state of sulphur in ${{H}_{2}}S{{O}_{5}}$,
If we find oxidation state, consider sulphur as x, then it will be:
\[\begin{align}
& 2\times \left( 1 \right)+x+5\times \left( -2 \right)=0 \\
& 2+x+\left( -10 \right)=0 \\
& x=+8 \\
\end{align}\]
We get the oxidation state as +8, but this is not true, because the maximum oxidation number for sulphur cannot exceed +6. Since sulphur has only 6 electrons in its valence shell.
-We get this exceptional value of oxidation state for sulphur in${{H}_{2}}S{{O}_{5}}$ due to the peroxy linkage shown by two atoms.
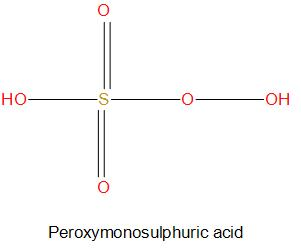
- We can see from the structure of ${{H}_{2}}S{{O}_{5}}$, there are two peroxide molecules present and all the other oxygen atoms are normally attached. Here we will consider the charge on three oxygen atoms as -2 and charge on two peroxide molecules as -1. So, if we find the oxidation state of sulphur, then we get:
\[\begin{align}
& 2\times \left( 1 \right)+2\times \left( -1 \right)+3\times \left( -2 \right)+x=0 \\
& 2+\left( -2 \right)+\left( -6 \right)+x=0 \\
& x=+6 \\
\end{align}\]
Hence, the oxidation state of ${{H}_{2}}S{{O}_{5}}$is +6.
- Let’s next find the oxidation state of sulphur in${{H}_{2}}{{S}_{2}}{{O}_{8}}$:
If we find oxidation state, consider sulphur as x, then it will be:
\[\begin{align}
& 2\times \left( 1 \right)+2\times x+8\times \left( -2 \right)=0 \\
& 2+2x+\left( -16 \right)=0 \\
& x=+7 \\
\end{align}\] \[\]
We get the oxidation state as +7, but this is not true, because the maximum oxidation number for sulphur cannot exceed +6. Since sulphur has only 6 electrons in its valence shell.
-We get this exceptional value of oxidation state for sulphur in ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ due to the peroxy linkage shown by two atoms.
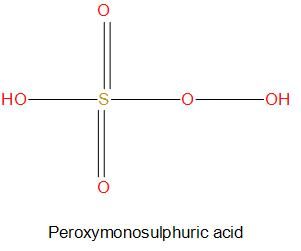
- We can see from the structure of ${{H}_{2}}{{S}_{2}}{{O}_{8}}$, there are two peroxide molecules present and all the other oxygen atoms are normally attached. Here we will consider the charge on six oxygen atoms as -2 and charge on two peroxide molecules as -1. So, if we find the oxidation state of sulphur, then we get:
\[\begin{align}
& 2\times \left( 1 \right)+2\times x+2\times \left( -1 \right)+6\times \left( -1 \right)=0 \\
& 2+2x+\left( -2 \right)+\left( -12 \right)=0 \\
& 2+2x-2-12=0 \\
& 2x=12 \\
& x=+6 \\
\end{align}\]
Hence, the oxidation state of ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ is +6.
Hence, the correct option is C.
That is the oxidation numbers of the sulphur atoms in peroxomonosulphuric acid ${{H}_{2}}S{{O}_{5}}$ and peroxodisulfuric acid ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ are respectively +6 and+6.
Note:
- It is to be noted here that there is peroxy linkage shown by two oxygen atoms, and therefore in such cases we have to consider the separate charge of two peroxide molecules and then should find the oxidation state of the central metal atoms.
To find the oxidation numbers we should know the charges present on each atom. Here, the compounds given are having peroxy linkage that is there is presence of a O-O single bond.
Complete step by step answer:
- Let’s first find the oxidation state of sulphur in ${{H}_{2}}S{{O}_{5}}$,
If we find oxidation state, consider sulphur as x, then it will be:
\[\begin{align}
& 2\times \left( 1 \right)+x+5\times \left( -2 \right)=0 \\
& 2+x+\left( -10 \right)=0 \\
& x=+8 \\
\end{align}\]
We get the oxidation state as +8, but this is not true, because the maximum oxidation number for sulphur cannot exceed +6. Since sulphur has only 6 electrons in its valence shell.
-We get this exceptional value of oxidation state for sulphur in${{H}_{2}}S{{O}_{5}}$ due to the peroxy linkage shown by two atoms.

- We can see from the structure of ${{H}_{2}}S{{O}_{5}}$, there are two peroxide molecules present and all the other oxygen atoms are normally attached. Here we will consider the charge on three oxygen atoms as -2 and charge on two peroxide molecules as -1. So, if we find the oxidation state of sulphur, then we get:
\[\begin{align}
& 2\times \left( 1 \right)+2\times \left( -1 \right)+3\times \left( -2 \right)+x=0 \\
& 2+\left( -2 \right)+\left( -6 \right)+x=0 \\
& x=+6 \\
\end{align}\]
Hence, the oxidation state of ${{H}_{2}}S{{O}_{5}}$is +6.
- Let’s next find the oxidation state of sulphur in${{H}_{2}}{{S}_{2}}{{O}_{8}}$:
If we find oxidation state, consider sulphur as x, then it will be:
\[\begin{align}
& 2\times \left( 1 \right)+2\times x+8\times \left( -2 \right)=0 \\
& 2+2x+\left( -16 \right)=0 \\
& x=+7 \\
\end{align}\] \[\]
We get the oxidation state as +7, but this is not true, because the maximum oxidation number for sulphur cannot exceed +6. Since sulphur has only 6 electrons in its valence shell.
-We get this exceptional value of oxidation state for sulphur in ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ due to the peroxy linkage shown by two atoms.

- We can see from the structure of ${{H}_{2}}{{S}_{2}}{{O}_{8}}$, there are two peroxide molecules present and all the other oxygen atoms are normally attached. Here we will consider the charge on six oxygen atoms as -2 and charge on two peroxide molecules as -1. So, if we find the oxidation state of sulphur, then we get:
\[\begin{align}
& 2\times \left( 1 \right)+2\times x+2\times \left( -1 \right)+6\times \left( -1 \right)=0 \\
& 2+2x+\left( -2 \right)+\left( -12 \right)=0 \\
& 2+2x-2-12=0 \\
& 2x=12 \\
& x=+6 \\
\end{align}\]
Hence, the oxidation state of ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ is +6.
Hence, the correct option is C.
That is the oxidation numbers of the sulphur atoms in peroxomonosulphuric acid ${{H}_{2}}S{{O}_{5}}$ and peroxodisulfuric acid ${{H}_{2}}{{S}_{2}}{{O}_{8}}$ are respectively +6 and+6.
Note:
- It is to be noted here that there is peroxy linkage shown by two oxygen atoms, and therefore in such cases we have to consider the separate charge of two peroxide molecules and then should find the oxidation state of the central metal atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




