
The pair that has similar shape is
A. \[B{F_3},Br{F_3}\]
B. \[{S_2}{F_2},{S_2}C{l_2}\]
C. \[{O_2}{F_2},{S_2}C{l_2}\]
D. \[{B_2}{H_6},{N_2}{H_4}\]
Answer
576.6k+ views
Hint: \[{S_2}{F_2}\] and \[{S_2}C{l_2}\] both have \[s{p^3}d\] hybridization and has the shape which is similar to \[{H_2}{O_2}\]. In the same way, \[{O_2}{F_2}\] has the structure similar to \[{H_2}{O_2}\].
Complete step by step answer:
\[B{F_3}\] have the \[s{p^2}\] hybridization but \[Br{F_3}\] (Bromine Trifluoride) have the \[s{p^3}d\] hybridization. So, \[B{F_3}\] is triangular (Trigonal planar) in shape having bond angle \[120^\circ \] but \[Br{F_3}\] is ‘T’ shaped or trigonal bipyramidal with a bond angle of \[86.2^\circ \].

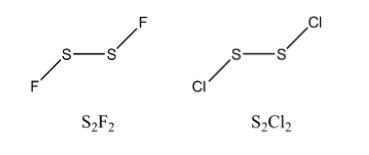
\[{S_2}{F_2}\] (Disulphuric difluoride) is a halide of sulphur have the \[s{p^3}{d^{}}\] hybridization. Its shape is gauche (half-open book shape) which is similar to \[{H_2}{O_2}\] with bond angle of \[108.3^\circ .{S_2}C{l_2}\] is a polar molecule and has \[s{p^3}d\] hybridization. It has the shape of gauche.
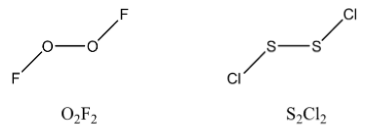
\[{O_2}{F_2}\] has the structure similar to \[{H_2}{O_2}\]. It has the shape of a half-open book (gauche in shape) \[{S_2}C{l_2}\] also has the shape similar to \[{O_2}{F_2}\].
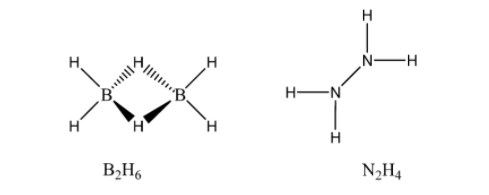
In (Diborane), Boron is \[s{p^3}\] hybridised, there are two terminals \[B - H\] bonds for each boron atom and there are only 12 bonding electrons available. The structure of Diborane molecule consists of 4 H atoms and that of two boron atoms coming on the same plane. In between these planes, there are said to be two dividing atoms of H. The two atoms of B left with that of each unpaired electron orbital and empty orbital forms the two bridging’s \[\left( {B-H-B} \right)\] bonds with that of the two 1s hydrogen atoms, is also called as the banana bond and on the other hand, \[{N_2}{H_4}\] (Nitrogen hydride or Hydrazine) is a strong base. Each subunit of \[{H_2}N - N\] is pyramidal and \[N - N\] bond distance is about \[1.4A^\circ \].
Therefore, the correct answer is option (B) and (C).
Note: Diborane is a chemical compound that consists of boron (B) and hydrogen (H) atoms and has a molecular formula \[{B_2}{H_6}\]. This substance is highly unstable at the room temperature and has a sweet odour. The compounds consisting of boron and hydrogen atoms are called boranes and diborane is one of the simplest boron hydrides.
Complete step by step answer:
\[B{F_3}\] have the \[s{p^2}\] hybridization but \[Br{F_3}\] (Bromine Trifluoride) have the \[s{p^3}d\] hybridization. So, \[B{F_3}\] is triangular (Trigonal planar) in shape having bond angle \[120^\circ \] but \[Br{F_3}\] is ‘T’ shaped or trigonal bipyramidal with a bond angle of \[86.2^\circ \].

\[{S_2}{F_2}\] (Disulphuric difluoride) is a halide of sulphur have the \[s{p^3}{d^{}}\] hybridization. Its shape is gauche (half-open book shape) which is similar to \[{H_2}{O_2}\] with bond angle of \[108.3^\circ .{S_2}C{l_2}\] is a polar molecule and has \[s{p^3}d\] hybridization. It has the shape of gauche.

\[{O_2}{F_2}\] has the structure similar to \[{H_2}{O_2}\]. It has the shape of a half-open book (gauche in shape) \[{S_2}C{l_2}\] also has the shape similar to \[{O_2}{F_2}\].

In (Diborane), Boron is \[s{p^3}\] hybridised, there are two terminals \[B - H\] bonds for each boron atom and there are only 12 bonding electrons available. The structure of Diborane molecule consists of 4 H atoms and that of two boron atoms coming on the same plane. In between these planes, there are said to be two dividing atoms of H. The two atoms of B left with that of each unpaired electron orbital and empty orbital forms the two bridging’s \[\left( {B-H-B} \right)\] bonds with that of the two 1s hydrogen atoms, is also called as the banana bond and on the other hand, \[{N_2}{H_4}\] (Nitrogen hydride or Hydrazine) is a strong base. Each subunit of \[{H_2}N - N\] is pyramidal and \[N - N\] bond distance is about \[1.4A^\circ \].

Therefore, the correct answer is option (B) and (C).
Note: Diborane is a chemical compound that consists of boron (B) and hydrogen (H) atoms and has a molecular formula \[{B_2}{H_6}\]. This substance is highly unstable at the room temperature and has a sweet odour. The compounds consisting of boron and hydrogen atoms are called boranes and diborane is one of the simplest boron hydrides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




