
The pH of the digestive juices within the human small intestine is between 7.5 and 8.5. This environment is slightly
a) Basic
b) Acidic
c) Neutral
d) None of the above
Answer
540.6k+ views
Hint: pH 7.5-8.5 is a high pH value which corresponds to low $H^{+}$ ions. Also, acidic and basic environments have high and low concentrations of $H^{+}$ ions respectively.
Complete answer:
The human small intestine has a slightly basic environment with the pH of the digestive juices between 7.5 and 8.5. The quantitative measure of the acidity and basicity of an aqueous solution is termed as pH. It refers to the relative concentration of $H^+$ ions in a solution. Low pH values indicate high concentrations of $H^+$ ions (acids) and high pH values indicate low concentrations.
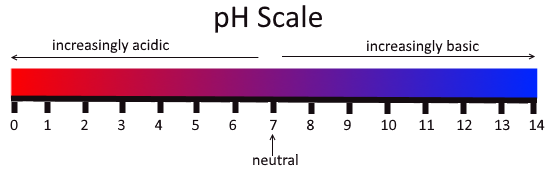
Fig: The pH scale
The values of the concentration of the hydrogen ion ranging between about 1 and $10^{-14}$ gram-equivalents per liter alternately between 0 and 14 are defined by the pH. In pure water the pH is 7 that means the concentration of the hydrogen ion is $10^{-7}$ gram-equivalents per liter. Pure water, pH 7 is neither acidic nor basic, it is neutral. A solution with a pH less than 7 is considered acidic whereas a solution with a pH greater than 7 is considered basic, or alkaline. A scale which measures from 0 to 14, and indicates just how acidic or basic a substance is is called pH scale. The pH of a substance is given by
$pH = −log [H^{+}]$
$pOH = −log [OH^{-}]$
where $[H^{+}]$denotes the molar hydrogen ion concentration,
$[OH^{-}]$ denotes the molar hydroxide ion concentration
Also, $pH + pOH = 14$
So, the correct answer is ‘Basic’.
Note:
- pH stands for potential of hydrogen.
- Danish biochemist Soren Peter Lauritz Sorensen devised the pH scale in 1923.
Complete answer:
The human small intestine has a slightly basic environment with the pH of the digestive juices between 7.5 and 8.5. The quantitative measure of the acidity and basicity of an aqueous solution is termed as pH. It refers to the relative concentration of $H^+$ ions in a solution. Low pH values indicate high concentrations of $H^+$ ions (acids) and high pH values indicate low concentrations.

Fig: The pH scale
The values of the concentration of the hydrogen ion ranging between about 1 and $10^{-14}$ gram-equivalents per liter alternately between 0 and 14 are defined by the pH. In pure water the pH is 7 that means the concentration of the hydrogen ion is $10^{-7}$ gram-equivalents per liter. Pure water, pH 7 is neither acidic nor basic, it is neutral. A solution with a pH less than 7 is considered acidic whereas a solution with a pH greater than 7 is considered basic, or alkaline. A scale which measures from 0 to 14, and indicates just how acidic or basic a substance is is called pH scale. The pH of a substance is given by
$pH = −log [H^{+}]$
$pOH = −log [OH^{-}]$
where $[H^{+}]$denotes the molar hydrogen ion concentration,
$[OH^{-}]$ denotes the molar hydroxide ion concentration
Also, $pH + pOH = 14$
So, the correct answer is ‘Basic’.
Note:
- pH stands for potential of hydrogen.
- Danish biochemist Soren Peter Lauritz Sorensen devised the pH scale in 1923.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




