
The pink color of Lithium chloride is due to:
A.Frenkel defect
B.Metal excess defect
C.Metal deficiency defect
D.Impurity defect
Answer
581.7k+ views
Hint: Crystals impart color due to various reasons such as the diffraction of light through the crystal structures, presence of imperfections or defects in crystals etc. This one deals with crystal defects.
Complete step by step answer:
Primarily, we need a very basic idea about crystals. Crystals are nothing but a perfectly ordered array of atoms or molecules or ions forming a crystal lattice. Let’s see a perfect crystal.
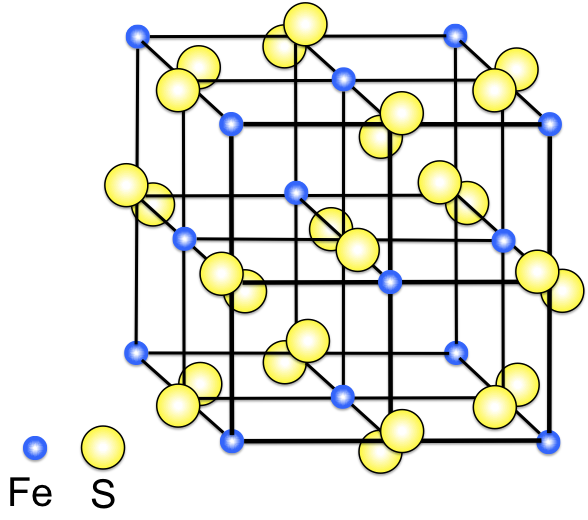
Since, all the available lattice positions are occupied in an ordered manner, hence it can be called as a perfect crystal.
But as we know, things are seldom perfect. Same goes for crystals. There are some kinds of imperfections present in crystals. And this is known as crystal defect. And this defect in turn leads to the color of the crystals. Let’s see how the crystal defect looks like.
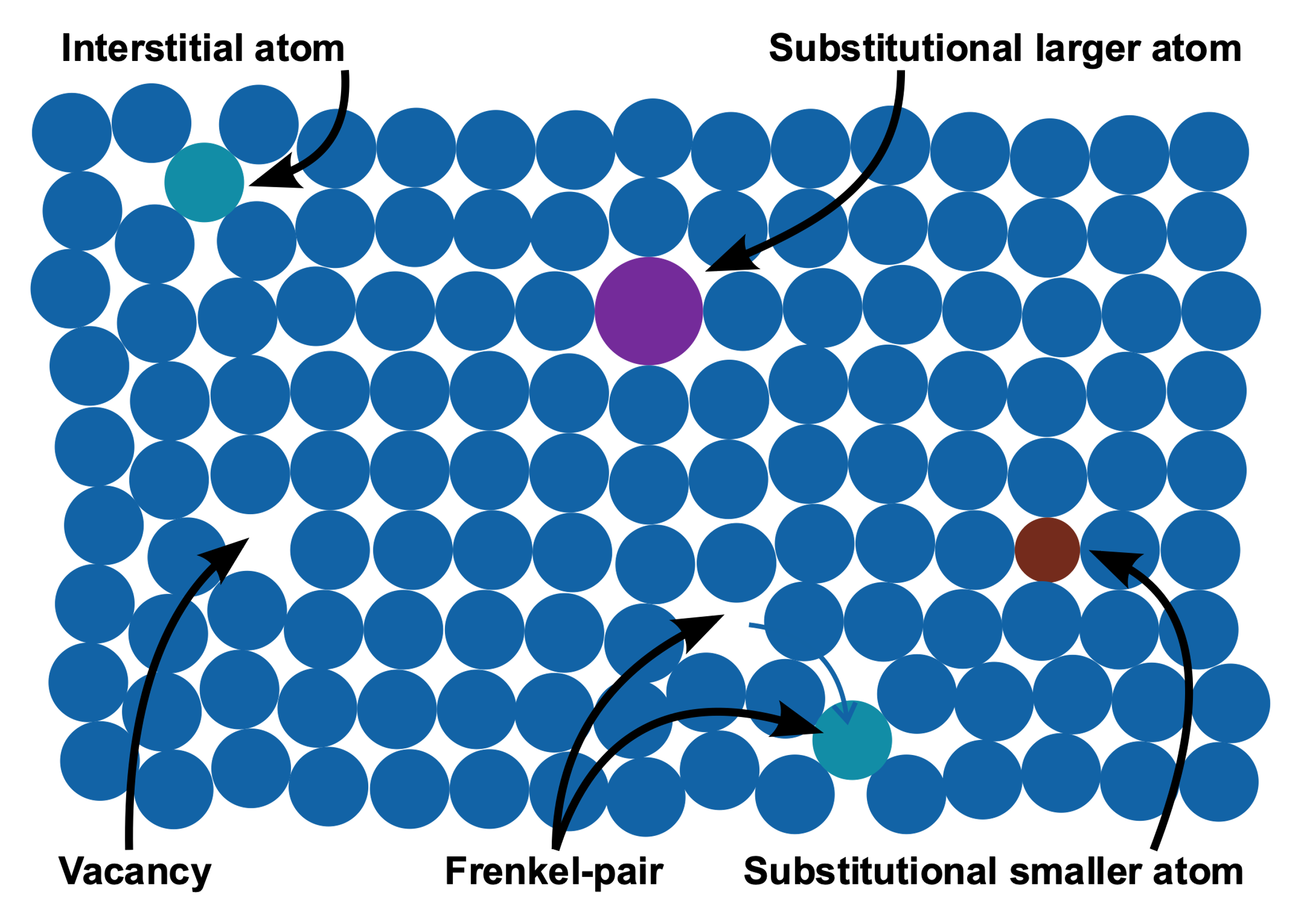
From the above picture we can easily identify some defects such as vacant positions, substitution by larger or smaller atoms etc.
In \[LiCl\], some of the \[C{l^ - }\] ions are actually missing from the lattice site leaving holes. And to maintain electrical neutrality, these vacancy sites or holes are occupied by electrons. One thing is that if a site of an anion is replaced by electrons, then these sites are known as F-centre. So, what happens is that when light energy falls on the crystal, these electrons are excited due to the absorption of energy & we see the corresponding complementary pink color.
And since, \[C{l^ - }\] ions are missing from the lattice, hence we can also say that excess metals are present in the crystal. So, you can easily guess that it is actually a metal excess defect.
So, the correct answer is Option B .
Note:
You might think that since ‘defect’ is a negative term then it must be undesirable for a crystal to have defects. But in reality, defects are useful in many cases. Crystal defects are important to impart semi-conductor property in a material and it is used in computer chips & other electronic devices.
Complete step by step answer:
Primarily, we need a very basic idea about crystals. Crystals are nothing but a perfectly ordered array of atoms or molecules or ions forming a crystal lattice. Let’s see a perfect crystal.

Since, all the available lattice positions are occupied in an ordered manner, hence it can be called as a perfect crystal.
But as we know, things are seldom perfect. Same goes for crystals. There are some kinds of imperfections present in crystals. And this is known as crystal defect. And this defect in turn leads to the color of the crystals. Let’s see how the crystal defect looks like.

From the above picture we can easily identify some defects such as vacant positions, substitution by larger or smaller atoms etc.
In \[LiCl\], some of the \[C{l^ - }\] ions are actually missing from the lattice site leaving holes. And to maintain electrical neutrality, these vacancy sites or holes are occupied by electrons. One thing is that if a site of an anion is replaced by electrons, then these sites are known as F-centre. So, what happens is that when light energy falls on the crystal, these electrons are excited due to the absorption of energy & we see the corresponding complementary pink color.
And since, \[C{l^ - }\] ions are missing from the lattice, hence we can also say that excess metals are present in the crystal. So, you can easily guess that it is actually a metal excess defect.
So, the correct answer is Option B .
Note:
You might think that since ‘defect’ is a negative term then it must be undesirable for a crystal to have defects. But in reality, defects are useful in many cases. Crystal defects are important to impart semi-conductor property in a material and it is used in computer chips & other electronic devices.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




