
The positive charge on proton ……………
A. Is double of the negative charge on the electron.
B. Is half of the negative charge on the electron.
C. Is equal to the negative charge on the electron.
D. Has no relation with the charge on an electron.
Answer
596.7k+ views
Hint: The number of positive charge particle protons and negative charge particle electrons are equal in an atom. Electrons and protons are opposite of each other. Charge is different from the mass of the proton.
Complete step by step answer:
Physicist Ernest Rutherford has discovered the nucleus and the protons and neutrons contribute the equal share to form an atom. The nucleus has been bounded with the presence of strong force and it reduced the creepy electric force between proton and neutron particles like carbon-14 crumbling into nitrogen-14.
Through cathode ray tube experiment, Rutherford has discovered protons and it is a positively charged particle. Protons are 99.86% greater than neutrons. The number of protons in an atom is equal to the atomic number of particular atoms. Neutrons were invented by Chadwick in 1932 and it is a neutral charge particle. It is also made up of quarks.
Electrons are 1800 times tiny than protons or neutrons and it is discovered by John Thompson. It has a negatively charged particle and moves the orbit of the atom.
Electrons have ‘-1’ or ‘0’ electric charge whereas protons have ‘+1’ or ‘0’ electric charge on it. So, the charge on the electron and proton cancels out each other hence the atom is electrically neutral in nature. Protons are present inside the nucleus in an atom whereas electrons are found on the outside of the nucleus.
Hence the positive charge on proton is equal to the negative charge on electron for any specified atom.
So, the correct option is C.
Additional Information:
Atoms are the primary entities of matter and the prescribing structure of elements. It is formed with three particles – electrons, protons and neutrons and can be made with even smaller particles i.e. quarks. According to the CERN experiment of universe existence, quarks have the ability to form protons and neutrons which is combined into nuclei.
There are many models of atom discovered by any scientists like:
- Dalton's model of the atom
- Thomson's model of the atom
- Rutherford's model of the atom
- Bohr's model of the atom
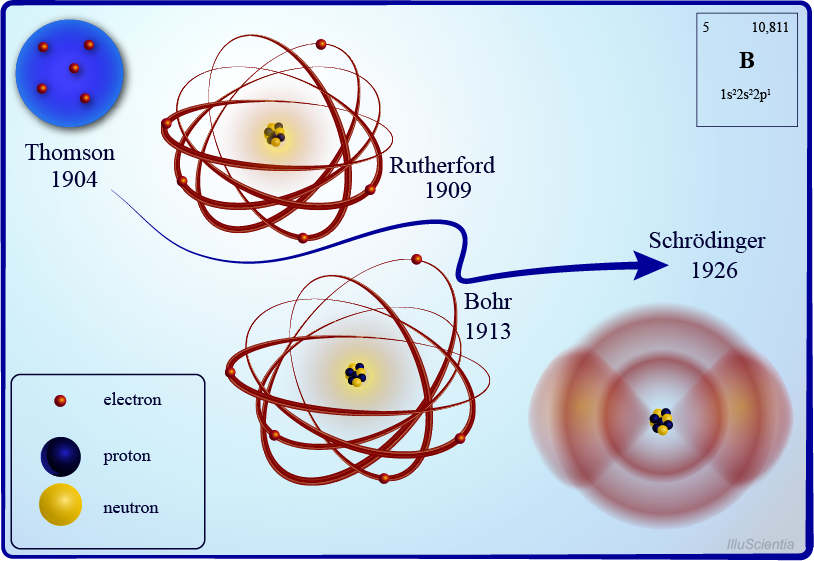
Note: The charge on proton is equal and opposite to the charge on electron. And neutrons are neutral in nature. Remember an atom is made up of three particles i.e., proton, electron and neutron. And the electrical charge of protons is always one, i.e., ‘+1’.
The mass of proton is one atomic mass which is equal to $1.67\times 10^{-27}$
Complete step by step answer:
Physicist Ernest Rutherford has discovered the nucleus and the protons and neutrons contribute the equal share to form an atom. The nucleus has been bounded with the presence of strong force and it reduced the creepy electric force between proton and neutron particles like carbon-14 crumbling into nitrogen-14.
Through cathode ray tube experiment, Rutherford has discovered protons and it is a positively charged particle. Protons are 99.86% greater than neutrons. The number of protons in an atom is equal to the atomic number of particular atoms. Neutrons were invented by Chadwick in 1932 and it is a neutral charge particle. It is also made up of quarks.
Electrons are 1800 times tiny than protons or neutrons and it is discovered by John Thompson. It has a negatively charged particle and moves the orbit of the atom.
Electrons have ‘-1’ or ‘0’ electric charge whereas protons have ‘+1’ or ‘0’ electric charge on it. So, the charge on the electron and proton cancels out each other hence the atom is electrically neutral in nature. Protons are present inside the nucleus in an atom whereas electrons are found on the outside of the nucleus.
Hence the positive charge on proton is equal to the negative charge on electron for any specified atom.
So, the correct option is C.
Additional Information:
Atoms are the primary entities of matter and the prescribing structure of elements. It is formed with three particles – electrons, protons and neutrons and can be made with even smaller particles i.e. quarks. According to the CERN experiment of universe existence, quarks have the ability to form protons and neutrons which is combined into nuclei.
There are many models of atom discovered by any scientists like:
- Dalton's model of the atom
- Thomson's model of the atom
- Rutherford's model of the atom
- Bohr's model of the atom

Note: The charge on proton is equal and opposite to the charge on electron. And neutrons are neutral in nature. Remember an atom is made up of three particles i.e., proton, electron and neutron. And the electrical charge of protons is always one, i.e., ‘+1’.
The mass of proton is one atomic mass which is equal to $1.67\times 10^{-27}$
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Which Country is Called "The Land of Festivals"?

What type of cell is found in the Seminiferous tub class 10 biology CBSE

What are the public facilities provided by the government? Also explain each facility




