
The presence of radiation in a gas discharge discovered by E.Goldstein is called as:
A.Canal rays
B.Electrons
C.Subatomic particles.
D.Protons.
Answer
574.2k+ views
Hint: We should know about the major discoveries and names of the scientist who led to those discoveries.For example, Chadwick discovered the presence of neutrons in an atom. Similarly, electrons and protons are also discovered by eminent scientists.
Complete step by step answer:
E.Goldstein performed an experiment in which he discovered radiations named canal rays. In other words, we know this as the discovery of protons. In board examination, we can get this question in three different ways. Firstly as explain the Goldstein experiment. Secondly, discuss the discovery of protons in your words. Thirdly, we can find the question regarding canal rays. Since, E.Goldstein discovered these rays, the experiment is named after him.
Let us understand it diagrammatically.
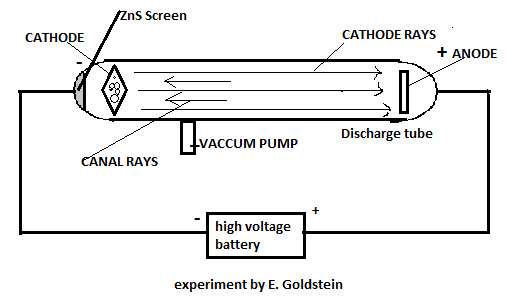
He discovered, along with cathode rays, there are certain types of radiations travelling in the opposite direction to that of cathode rays and termed them as canal rays. J.J.Thomson also performed a similar experiment where he discovered the cathode rays.
The difference between the two experiments is that E.Goldstein used perforated cathode. In layman language, we can say that the cathode was having holes.
In this, he maintained a potential of 10000volt and at very low pressure, he observed that Cathode rays were travelling from cathode to anode, whereas some of the rays were moving towards cathode and were leading to the phenomenon of fluorescence on the discharge tube. Discharge tube was coated with zinc sulphide (\[ZnS\]) and scintillation was observed.
Let us take hydrogen gas in the discharge tube. On applying the potential across the system, the electron in the atom started moving towards anode and the positively charged ion moved towards the cathode.
Properties:
-Canal rays travel in a straight line.
-Shows deflection in the presence of electric and magnetic fields.
-These rays are positively charged particles and carry the charge = \[1.0622 \times {10^{ - 19}}C\]
-They have the ability to cause fluorescence when they strike the \[ZnS\] coated screen.
-Charge is to mass ratio e/m= \[9.6 \times {10^7}CperKg\], it depends upon the gas taken in the discharge tube.
Mass = \[1.6726 \times {10^{ - 27}}kg\]
-It travels opposite to that of the cathode rays.
-These rays have the ability to ionise the gas taken in the discharge tube.
-Depends on the nature of gas taken in the discharge tube.
Therefore, if the radiations are asked, we will mark canal rays as the correct answer. If the particle discovered is asked, it will be proton.
Hence optionA.(canal rays) is the correct answer.
Additional information:
From this experiment, we conclude that protons are much heavier than electrons.
The charge on a proton is the same as that of a negative charge on the electron.
Protons, like an electron, are part of every atom.
Note:
These canal rays are also called as channel rays because these were produced by perforation or holes or channels present on the cathode. In other terms, the canal rays are the positively charged radiations that led to the discovery of protons. Proton is considered to be a subatomic particle that is positively charged.
Complete step by step answer:
E.Goldstein performed an experiment in which he discovered radiations named canal rays. In other words, we know this as the discovery of protons. In board examination, we can get this question in three different ways. Firstly as explain the Goldstein experiment. Secondly, discuss the discovery of protons in your words. Thirdly, we can find the question regarding canal rays. Since, E.Goldstein discovered these rays, the experiment is named after him.
Let us understand it diagrammatically.

He discovered, along with cathode rays, there are certain types of radiations travelling in the opposite direction to that of cathode rays and termed them as canal rays. J.J.Thomson also performed a similar experiment where he discovered the cathode rays.
The difference between the two experiments is that E.Goldstein used perforated cathode. In layman language, we can say that the cathode was having holes.
In this, he maintained a potential of 10000volt and at very low pressure, he observed that Cathode rays were travelling from cathode to anode, whereas some of the rays were moving towards cathode and were leading to the phenomenon of fluorescence on the discharge tube. Discharge tube was coated with zinc sulphide (\[ZnS\]) and scintillation was observed.
Let us take hydrogen gas in the discharge tube. On applying the potential across the system, the electron in the atom started moving towards anode and the positively charged ion moved towards the cathode.
Properties:
-Canal rays travel in a straight line.
-Shows deflection in the presence of electric and magnetic fields.
-These rays are positively charged particles and carry the charge = \[1.0622 \times {10^{ - 19}}C\]
-They have the ability to cause fluorescence when they strike the \[ZnS\] coated screen.
-Charge is to mass ratio e/m= \[9.6 \times {10^7}CperKg\], it depends upon the gas taken in the discharge tube.
Mass = \[1.6726 \times {10^{ - 27}}kg\]
-It travels opposite to that of the cathode rays.
-These rays have the ability to ionise the gas taken in the discharge tube.
-Depends on the nature of gas taken in the discharge tube.
Therefore, if the radiations are asked, we will mark canal rays as the correct answer. If the particle discovered is asked, it will be proton.
Hence optionA.(canal rays) is the correct answer.
Additional information:
From this experiment, we conclude that protons are much heavier than electrons.
The charge on a proton is the same as that of a negative charge on the electron.
Protons, like an electron, are part of every atom.
Note:
These canal rays are also called as channel rays because these were produced by perforation or holes or channels present on the cathode. In other terms, the canal rays are the positively charged radiations that led to the discovery of protons. Proton is considered to be a subatomic particle that is positively charged.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




