
The presence or absence of a hydroxyl group in which carbon atom of sugar differentiates RNA and DNA?
A) 1st
B) 2nd
C) 3rd
D) 4th
Answer
566.7k+ views
Hint:To solve this question you must know the structure of DNA and RNA. Both DNA and RNA have a five membered ring to which the phosphate and a base is attached. The rest are either hydrogen or hydroxyl groups. After drawing the structure remember to name the carbon atoms starting from the base.
Complete step by step solution:
We know that RNA is ribonucleic acid and DNA is deoxyribonucleic acid. These are the two main types of nucleic acid. Now, let us discuss their chemical structures to answer the given question. Both RNA and DNA are made up of a nucleotide containing a five membered ring i.e. pentose sugar ring with a phosphate group and a nitrogen base. They look pretty similar but RNA is a single strand and DNA is a double stranded helix structure. Now let us draw their structures and see how DNA is different from RNA.
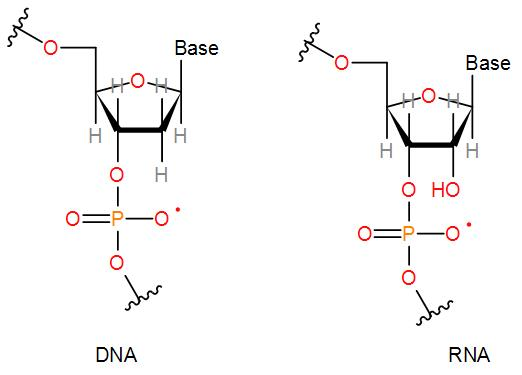
We can see from the above structures that both DNA and RNA have a base, a phosphate group and five-membered ring. The carbon attached to the base is carbon 1, that we can also write as ${{C}_{1}}$. Now, we can see that in RNA at the second carbon position that is ${{C}_{2}}$ there is a hydroxyl group present which is absent in the structure of DNA at the second carbon position. Therefore, the presence of the hydroxyl group in the second carbon atom of sugar differentiates RNA and DNA.
Therefore, the correct answer is option [B] 2nd
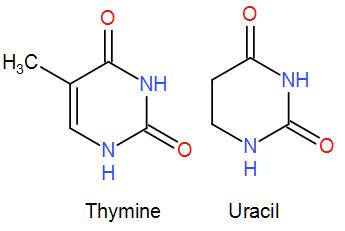
Note: The nitrogenous base found in RNA and DNA is also the same except for one base. In DNA we find cytosine, guanine, thymine and adenine base. Whereas in RNA, thymine is replaced by uracil and the rest of the bases are the same. The structure of thymine and uracil is pretty same except the presence of one methyl group in thymine and none in uracil.
Complete step by step solution:
We know that RNA is ribonucleic acid and DNA is deoxyribonucleic acid. These are the two main types of nucleic acid. Now, let us discuss their chemical structures to answer the given question. Both RNA and DNA are made up of a nucleotide containing a five membered ring i.e. pentose sugar ring with a phosphate group and a nitrogen base. They look pretty similar but RNA is a single strand and DNA is a double stranded helix structure. Now let us draw their structures and see how DNA is different from RNA.

We can see from the above structures that both DNA and RNA have a base, a phosphate group and five-membered ring. The carbon attached to the base is carbon 1, that we can also write as ${{C}_{1}}$. Now, we can see that in RNA at the second carbon position that is ${{C}_{2}}$ there is a hydroxyl group present which is absent in the structure of DNA at the second carbon position. Therefore, the presence of the hydroxyl group in the second carbon atom of sugar differentiates RNA and DNA.
Therefore, the correct answer is option [B] 2nd
Note: The nitrogenous base found in RNA and DNA is also the same except for one base. In DNA we find cytosine, guanine, thymine and adenine base. Whereas in RNA, thymine is replaced by uracil and the rest of the bases are the same. The structure of thymine and uracil is pretty same except the presence of one methyl group in thymine and none in uracil.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




