
The process of electrolysis is used in:
A.Extraction of metals
B.Electroplating
C.Refining of metals
D.All of the above
Answer
582.3k+ views
Hint: Here you need to think about the process of electrolysis. Electrolysis is the process in which we can get pure metals from its impure form.
Step by step answer: To answer this question we need to know everything about electrolysis. Let’s first start with the definition of electrolysis. Electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. It is used for separation of elements from naturally occurring sources such as ores using electrolytic cells. Decomposition potential is the voltage that is needed for electrolysis to occur.
Given below is the diagram of the process of electrolysis.
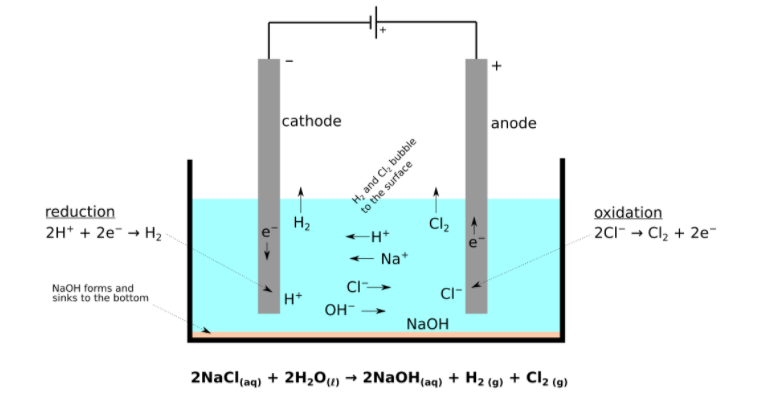
Process of Electrolysis:
The term and the process was invented by Michael Faraday in the 19th century. It was the process that helped in the study of chemical reactions for obtaining pure elements. The main components of this process is electrolyte, two electrodes (anode and cathode) and an external power source.
According to Faraday’s laws of electrolysis, the mass of elements deposited at an electrode is directly proportional to the charge in coulombs.
Here in the above diagram you can see that the electrolyte used is concentrated Sodium Chloride solution.
The anode is an impure rod of sodium and a cathode is a pure rod of sodium. When electricity is passed through the electrolyte and the electrodes, hydrogen gas is formed at the negative electrode and chlorine gas is formed at the positive electrode and a solution of sodium hydroxide is also formed. In this case, sodium is not deposited on cathode because it is very reactive and therefore forms Sodium hydroxide solution which is further used for making soaps, paper, bleach etc.
This is how extraction of sodium metal takes place and it is also called refining of metals. In some other cases metals get deposited on cathode i.e. on other blocks of metal which is called as electroplating.
From this whole process we get to know that all the answers are correct for the process of electrolysis.
Therefore, option D (all of the above) is the correct answer.
Note: If you know the process you will easily be able to answer this question. Some overview can also help answering this question as it is simple and direct question
Step by step answer: To answer this question we need to know everything about electrolysis. Let’s first start with the definition of electrolysis. Electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. It is used for separation of elements from naturally occurring sources such as ores using electrolytic cells. Decomposition potential is the voltage that is needed for electrolysis to occur.
Given below is the diagram of the process of electrolysis.

Process of Electrolysis:
The term and the process was invented by Michael Faraday in the 19th century. It was the process that helped in the study of chemical reactions for obtaining pure elements. The main components of this process is electrolyte, two electrodes (anode and cathode) and an external power source.
According to Faraday’s laws of electrolysis, the mass of elements deposited at an electrode is directly proportional to the charge in coulombs.
Here in the above diagram you can see that the electrolyte used is concentrated Sodium Chloride solution.
The anode is an impure rod of sodium and a cathode is a pure rod of sodium. When electricity is passed through the electrolyte and the electrodes, hydrogen gas is formed at the negative electrode and chlorine gas is formed at the positive electrode and a solution of sodium hydroxide is also formed. In this case, sodium is not deposited on cathode because it is very reactive and therefore forms Sodium hydroxide solution which is further used for making soaps, paper, bleach etc.
This is how extraction of sodium metal takes place and it is also called refining of metals. In some other cases metals get deposited on cathode i.e. on other blocks of metal which is called as electroplating.
From this whole process we get to know that all the answers are correct for the process of electrolysis.
Therefore, option D (all of the above) is the correct answer.
Note: If you know the process you will easily be able to answer this question. Some overview can also help answering this question as it is simple and direct question
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




