
The product obtained by reduction of benzyl bromide with \[{\text{LiAl}}{{\text{H}}_4}\] is
A)
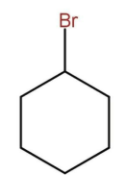
B)
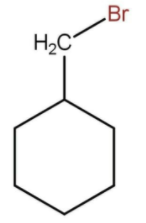
C)
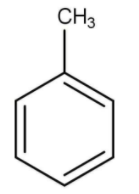
D)
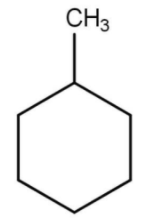




Answer
548.4k+ views
Hint:
An aromatic is formed out of reduction. The compound formed is colourless and water insoluble liquid. It is a mono substituted benzene derivative.
Complete step by step solution:
The reduction represents the addition of hydrogen in a molecule. When reduction of benzyl bromide in the presence of lithium aluminium hydride adds up the hydrogen in the methyl group and the formation of toluene occurs the reaction occurs as follow:
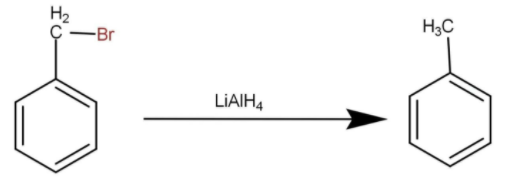
Hence, the correct option is C.
Additional information:
Lithium aluminium hydride is commonly known as LAH. It is an inorganic gray coloured solid. It is a very strong reducing agent. It is extensively used for the reduction of esters, carboxylic acid and amide. It reacts dangerously with water releasing hydrogen. It is a potential source for storage of hydrogen gas. Benzyl bromide is an organic compound. In benzyl bromide one hydrogen atom in the benzene ring is substituted with bromo methyl group. It is a colourless liquid. It is used in the organic synthesis of various benzyl derivatives. Toluene is used for various reactions such as nitration and oxidation. It is used as a solvent for paint, paint thinner and many chemical reactions, printing inks, adhesives, liquors and disinfectants. Toluene is also used as a fuel or as an additive in fuel.
Note:
Reduction and oxidation are simultaneous processes. If one substance is getting reduced then another substance will be getting oxidised for sure. For example the reduction of benzyl bromide occurs due to the addition of hydrogen, then simultaneously lithium aluminium hydride is getting oxidised.
An aromatic is formed out of reduction. The compound formed is colourless and water insoluble liquid. It is a mono substituted benzene derivative.
Complete step by step solution:
The reduction represents the addition of hydrogen in a molecule. When reduction of benzyl bromide in the presence of lithium aluminium hydride adds up the hydrogen in the methyl group and the formation of toluene occurs the reaction occurs as follow:

Hence, the correct option is C.
Additional information:
Lithium aluminium hydride is commonly known as LAH. It is an inorganic gray coloured solid. It is a very strong reducing agent. It is extensively used for the reduction of esters, carboxylic acid and amide. It reacts dangerously with water releasing hydrogen. It is a potential source for storage of hydrogen gas. Benzyl bromide is an organic compound. In benzyl bromide one hydrogen atom in the benzene ring is substituted with bromo methyl group. It is a colourless liquid. It is used in the organic synthesis of various benzyl derivatives. Toluene is used for various reactions such as nitration and oxidation. It is used as a solvent for paint, paint thinner and many chemical reactions, printing inks, adhesives, liquors and disinfectants. Toluene is also used as a fuel or as an additive in fuel.
Note:
Reduction and oxidation are simultaneous processes. If one substance is getting reduced then another substance will be getting oxidised for sure. For example the reduction of benzyl bromide occurs due to the addition of hydrogen, then simultaneously lithium aluminium hydride is getting oxidised.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




