
The product obtained in the reaction of \[C{H_3}CON{H_2}\] and $NaN{O_2}/HCl$ is –
(A).$C{H_3}COOH$
(B) $C{H_3}COC{H_3}$
(C) $C{H_3}CO\mathop N\limits^ \oplus {H_3}\mathop C\limits^{\left( - \right)} l$
(D) $C{H_3}N{H_2}$
Answer
587.4k+ views
Hint: Acetamide is an organic compound which releases nitrogen gas from the solution when reacted with $NaN{O_2}/HCl$.It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent.
Complete step by step answer:
We know the given compound \[C{H_3}CON{H_2}\] commonly known as acetamide is a organic compound with structure as: -
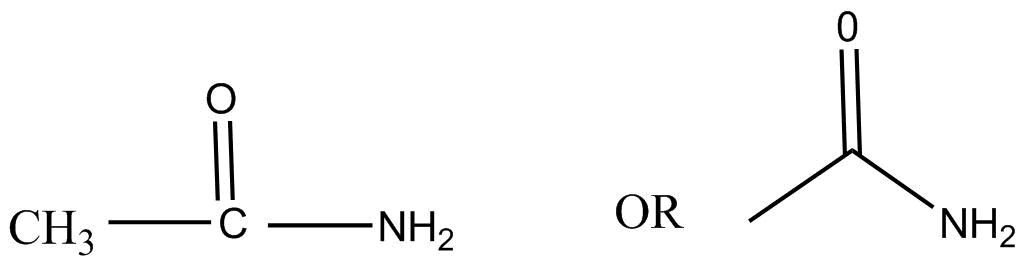
It is the simplest amide with IUPAC name ethanamide and is derived from acetic acid by the reaction with ammonia
$C{H_3}COOH(Acetic{\text{ }}Acid){\text{ }} + {\text{ }}H - N{H_2}{\text{ }}(Ammonia){\text{ }} \to {\text{ }}C{H_3}CON{H_2}{\text{ }}(Ethanamide)$
In industries, acetamide is produced by dehydrating ammonium acetate or via hydration of acetonitrile.
i.e. $C{H_3}CN + {H_2}O \to C{H_3}CON{H_2}$
Ethanamide is used as a plasticizer and an industrial solvent. It has uses in electro chemistry and organic synthesis of pharmaceuticals, pesticides and antioxidants for plastics. When $C{H_3}CON{H_2}$ is reacted with $NaN{o_2}$ in the presence of $HCl$, we get $C{H_3}COOH$(acetic acid) as the main product.
Note:
Acetic acid will also be formed from ethanamide if the reagent is $HN{O_2}$. Moreover, when ethanamide is treated with water, it also yields acetic acid.
i.e. $C{H_3}CoN{H_2} + {H_2}O \to C{H_3}COOH + {H_2}O$
Complete step by step answer:
We know the given compound \[C{H_3}CON{H_2}\] commonly known as acetamide is a organic compound with structure as: -
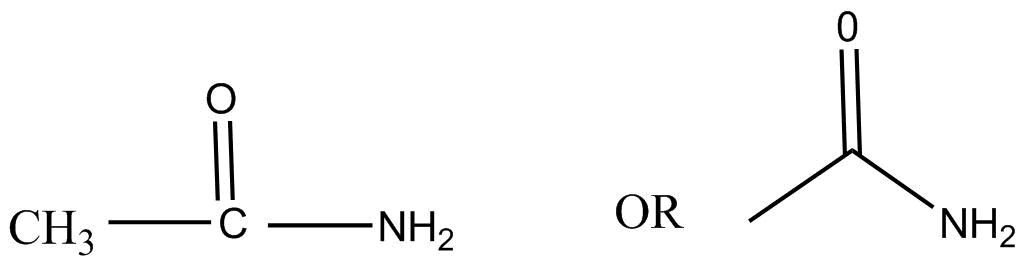
It is the simplest amide with IUPAC name ethanamide and is derived from acetic acid by the reaction with ammonia
$C{H_3}COOH(Acetic{\text{ }}Acid){\text{ }} + {\text{ }}H - N{H_2}{\text{ }}(Ammonia){\text{ }} \to {\text{ }}C{H_3}CON{H_2}{\text{ }}(Ethanamide)$
In industries, acetamide is produced by dehydrating ammonium acetate or via hydration of acetonitrile.
i.e. $C{H_3}CN + {H_2}O \to C{H_3}CON{H_2}$
Ethanamide is used as a plasticizer and an industrial solvent. It has uses in electro chemistry and organic synthesis of pharmaceuticals, pesticides and antioxidants for plastics. When $C{H_3}CON{H_2}$ is reacted with $NaN{o_2}$ in the presence of $HCl$, we get $C{H_3}COOH$(acetic acid) as the main product.
Note:
Acetic acid will also be formed from ethanamide if the reagent is $HN{O_2}$. Moreover, when ethanamide is treated with water, it also yields acetic acid.
i.e. $C{H_3}CoN{H_2} + {H_2}O \to C{H_3}COOH + {H_2}O$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




