
The product P for the above reaction will be ?
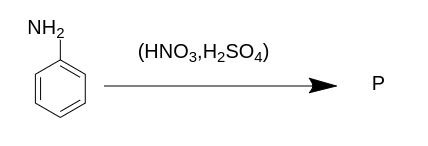
(A) m- nitroaniline
(B) o- nitroaniline
(C) p- nitroaniline
(D) both o and p - nitroaniline
Answer
515.4k+ views
Hint :To solve the given question, we should have knowledge about aniline, nitric acid and sulphuric acid. Aniline is an organic compound and has a phenyl group attached to an amine group and is the simplest aromatic amine. Nitric acid is a corrosive mineral acid which is colourless but later acquires a yellow colour due to decomposition. It is also known as aqua fortis.
Sulphuric acid is also a mineral acid consisting of elements like Sulphur, Oxygen and Hydrogen. It is also known as Oil of Vitriol. It is colourless, odourless and viscous liquid soluble with water at any concentration.
Complete Step By Step Answer:
Step-1 :
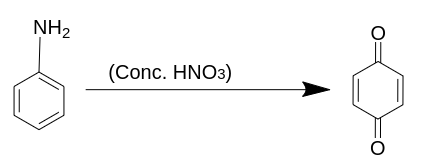
When concentrated nitric acid reacts with aniline, we get benzoquinone as an oxidised product.
Step-2 :
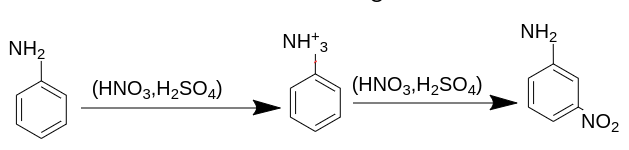
If we add concentrated acid along with sulphuric acid, we will get the intermediate as aniline ion, which contains positive charge and forms m - nitroaniline as a major product.
Note :
Aniline when treated with a mixture of concentrated sulphuric acid and nitric acid gives ortho , meta and para - nitroaniline as products with different percentage amounts. Aniline is an organic base and has different uses among household products like dyes, explosives, plastic, rubber and so on.
When we treat aniline with the mixture of nitric acid and sulphuric acid, we get $2% $ ortho product, para product and $51% $ meta product of nitroaniline.
Sulphuric acid is also a mineral acid consisting of elements like Sulphur, Oxygen and Hydrogen. It is also known as Oil of Vitriol. It is colourless, odourless and viscous liquid soluble with water at any concentration.
Complete Step By Step Answer:
Step-1 :
When concentrated nitric acid reacts with aniline, we get benzoquinone as an oxidised product.

Step-2 :
If we add concentrated acid along with sulphuric acid, we will get the intermediate as aniline ion, which contains positive charge and forms m - nitroaniline as a major product.

Note :
Aniline when treated with a mixture of concentrated sulphuric acid and nitric acid gives ortho , meta and para - nitroaniline as products with different percentage amounts. Aniline is an organic base and has different uses among household products like dyes, explosives, plastic, rubber and so on.
When we treat aniline with the mixture of nitric acid and sulphuric acid, we get $2% $ ortho product, para product and $51% $ meta product of nitroaniline.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




