
The Products (C) and (D) are:
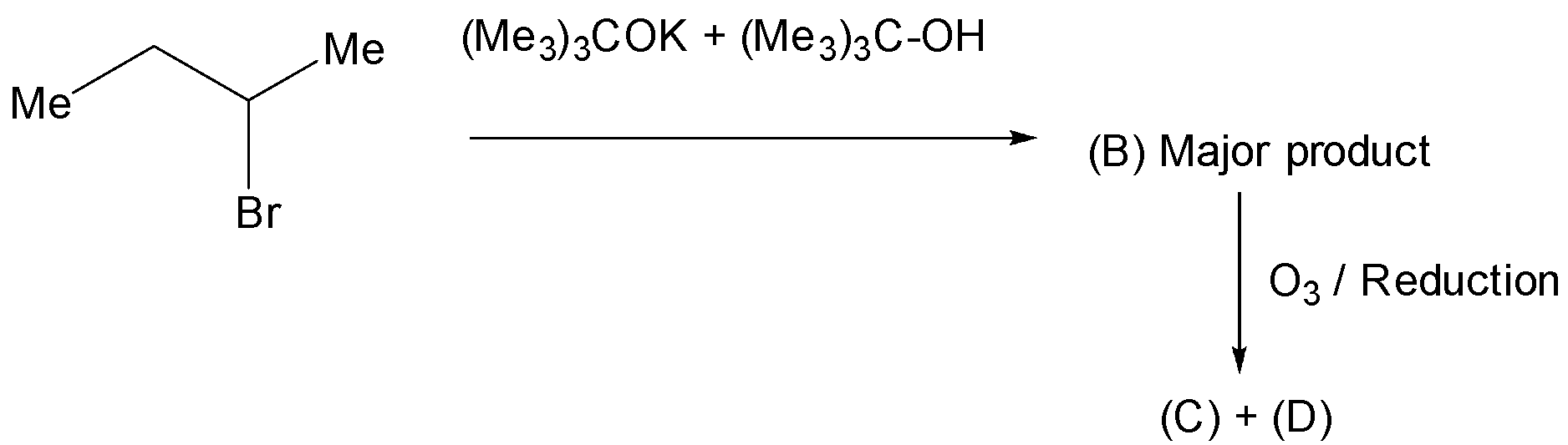
A.Methanal + Propanal
B.Propanoic acid + \[C{O_2}\]
C.2 mol ethanoic acid
D.2 mole ethanol
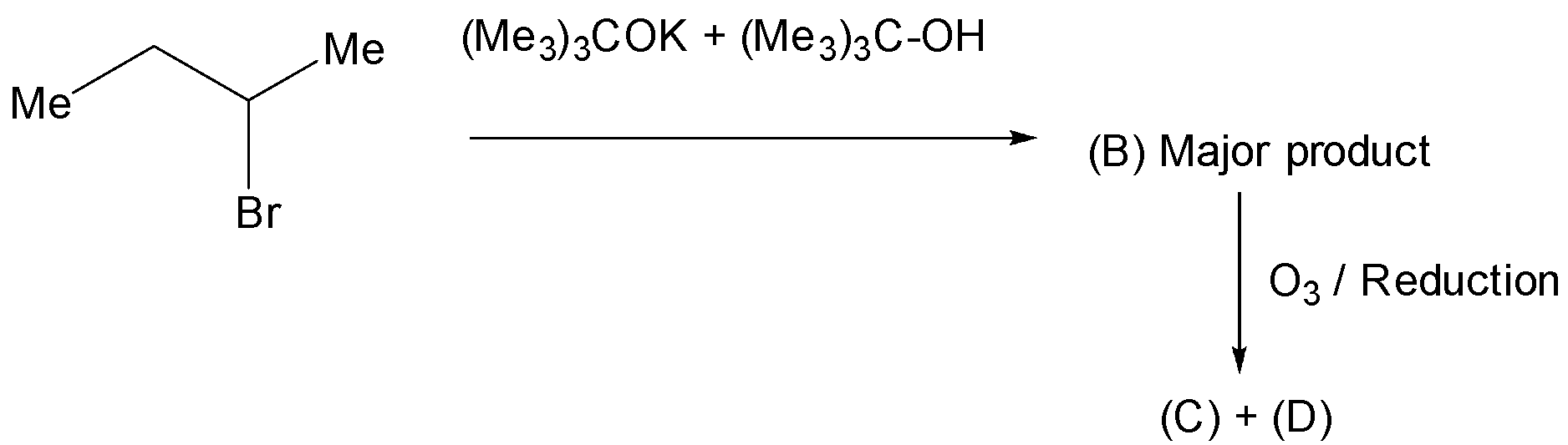
Answer
583.2k+ views
Hint: E2 elimination happens followed by ozonolysis.The strength of the base used in the reaction is more important for the elimination reactions.
Complete step by step solution:
Here the base used was potassium tert-butoxide which is a strong and bulky base. The proton from the carbon next to the halide ( \[\beta \] to halide) was abstracted and leads to the formation of olefin. The elimination is the concerted mechanism. The selectivity of picking protons from which \[\beta \] carbon is depends on the base. Less hindered bases are easily abstract the proton for the highly substituted carbon and yields the saytzeff's product as major product. Highly hindered bases are unable to pick the hydrogen from highly substituted carbon but easily from the primary carbon.
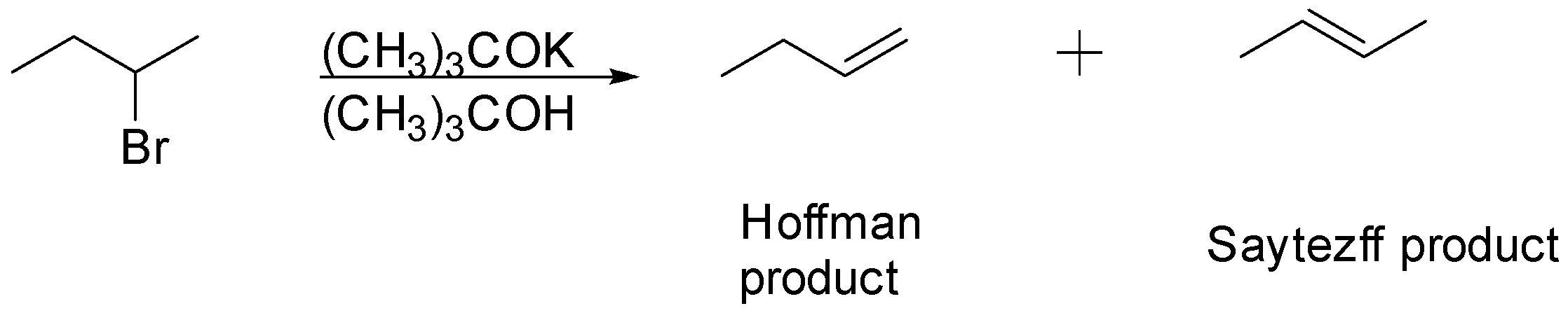
According to the Hofmann rule the less hindered proton could be easily picked up by the bulkier base and resulted in the less substituted alkene, kinetically controlled product. Whereas, the highly substituted alkene was formed as the major product while using a less hindered base.
The next step is the ozonolysis
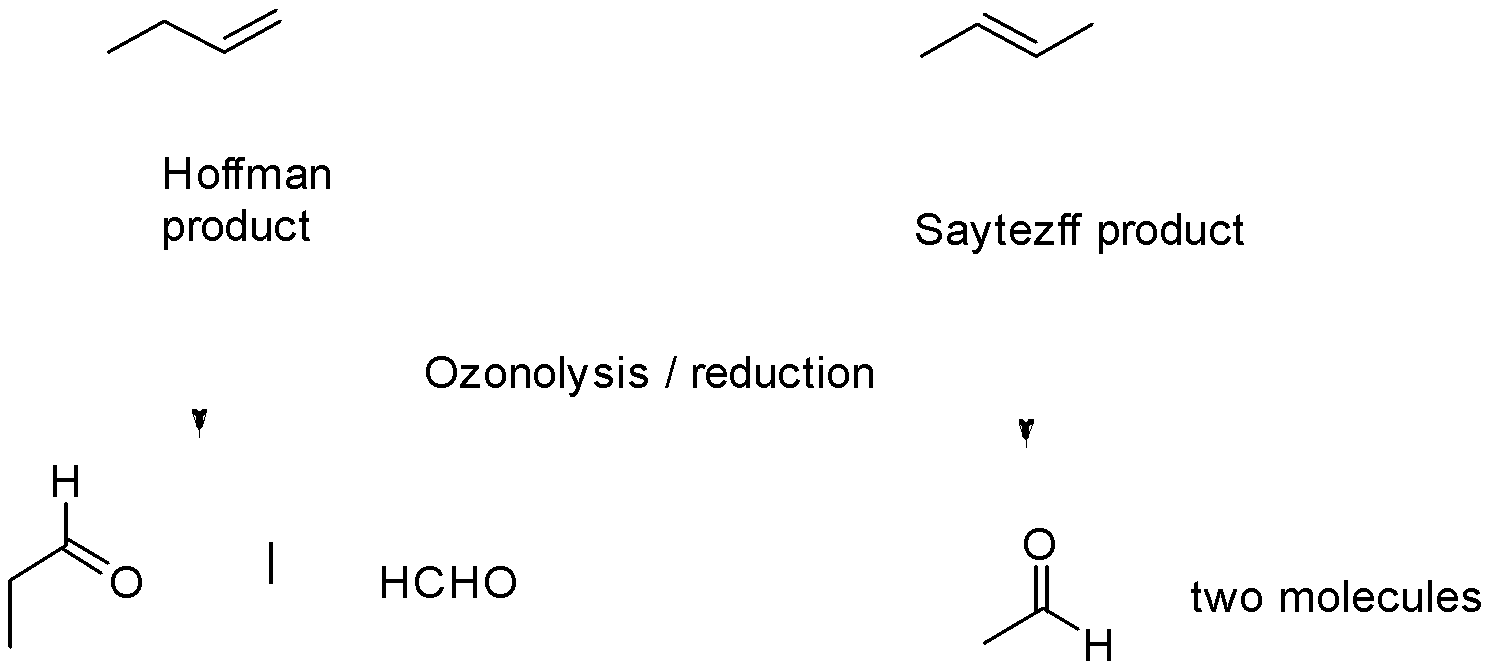
This step results with the cleavage of alkene by adding the ozone along the double bond.
Hence, the correct option is (A) i.e Methanol + Propanal
Note: Hofmann reaction is the reaction of bromine with sodium hydroxide forms sodium hypobromite. Initially base abstracts the acidic \[N - H\] proton. That will form an anion. This anion will react with bromine to give N-bromo amide.
Complete step by step solution:
Here the base used was potassium tert-butoxide which is a strong and bulky base. The proton from the carbon next to the halide ( \[\beta \] to halide) was abstracted and leads to the formation of olefin. The elimination is the concerted mechanism. The selectivity of picking protons from which \[\beta \] carbon is depends on the base. Less hindered bases are easily abstract the proton for the highly substituted carbon and yields the saytzeff's product as major product. Highly hindered bases are unable to pick the hydrogen from highly substituted carbon but easily from the primary carbon.

According to the Hofmann rule the less hindered proton could be easily picked up by the bulkier base and resulted in the less substituted alkene, kinetically controlled product. Whereas, the highly substituted alkene was formed as the major product while using a less hindered base.
The next step is the ozonolysis

This step results with the cleavage of alkene by adding the ozone along the double bond.
Hence, the correct option is (A) i.e Methanol + Propanal
Note: Hofmann reaction is the reaction of bromine with sodium hydroxide forms sodium hypobromite. Initially base abstracts the acidic \[N - H\] proton. That will form an anion. This anion will react with bromine to give N-bromo amide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




