
The product(s) show white ppt:
(A) Immediate
(B) After $ 5 $ min
(C) No ppt. at room temperature
(D) No reaction

Answer
535.5k+ views
Hint: Lucas reagent is a solution of anhydrous zinc chloride in the concentrated hydrochloric acid. This is basically the alcohol test. It will be helpful in determining the order of the alcohol whether it is primary, secondary or tertiary. The white ppt shows the presence of Cl negative ion that is the cream colored substance by adding the silver nitrate solution to any substance.
Complete step by step answer:
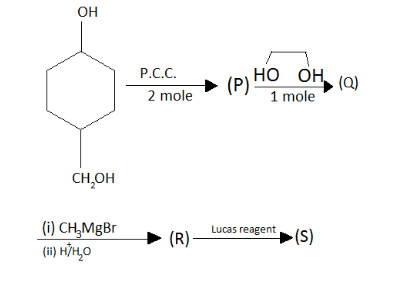
In the given question, coming to the first catalyst when PCC with two moles comes in the oxidation it will give;
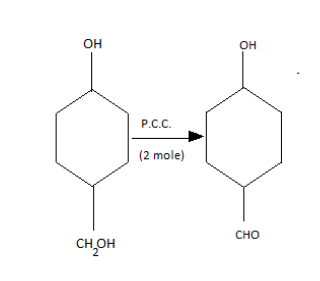
After this going to Q, catalyst with one mole then in the product, we get;
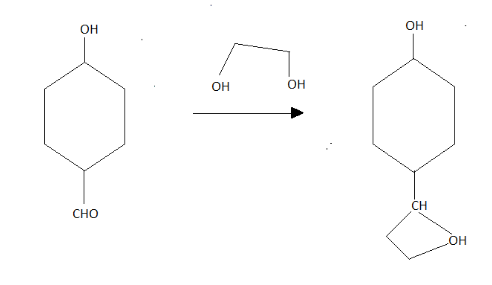
After this, going to Q to R here there are two catalysts . First one is the Grignard reagent and the second one is the H positive ion with water, the OH group will break out and the CH will become methyl group.
Lucas reagent is a solution of anhydrous zinc chloride in the concentrated hydrochloric acid. This is basically the alcohol test. It will be helpful in determining the order of the alcohol whether it is primary, secondary or tertiary.
Here when it react with R to give;
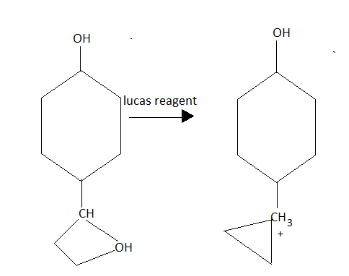
Here it is the third degree alcohol that is the tertiary alcohol. It is stable and will form the white ppt immediately as it gives turbidity.
Hence, the correct answer is option (A).
Note:
Chemical reaction involves chemical changes occurring within the substances, giving rise to a new element under certain conditions. The white ppt shows the presence of Cl negative ion that is the cream colored substance by adding the silver nitrate solution to any substance.
Complete step by step answer:
In the given question, coming to the first catalyst when PCC with two moles comes in the oxidation it will give;

After this going to Q, catalyst with one mole then in the product, we get;

After this, going to Q to R here there are two catalysts . First one is the Grignard reagent and the second one is the H positive ion with water, the OH group will break out and the CH will become methyl group.
Lucas reagent is a solution of anhydrous zinc chloride in the concentrated hydrochloric acid. This is basically the alcohol test. It will be helpful in determining the order of the alcohol whether it is primary, secondary or tertiary.
Here when it react with R to give;

Here it is the third degree alcohol that is the tertiary alcohol. It is stable and will form the white ppt immediately as it gives turbidity.
Hence, the correct answer is option (A).
Note:
Chemical reaction involves chemical changes occurring within the substances, giving rise to a new element under certain conditions. The white ppt shows the presence of Cl negative ion that is the cream colored substance by adding the silver nitrate solution to any substance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




