
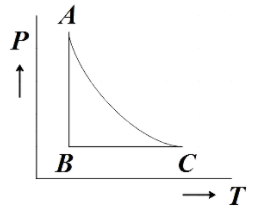
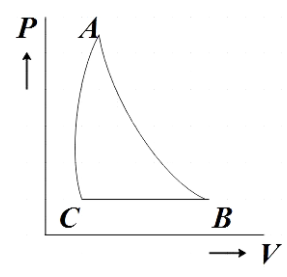
The PT diagram for an ideal gas is shown in the figure where AC is an adiabatic process. Find the corresponding P-V diagram in a figure.
A.
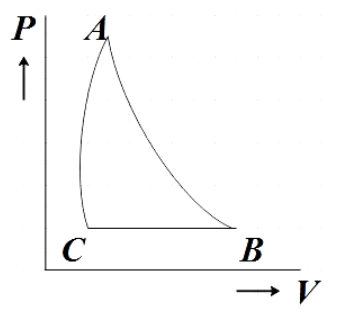
B.
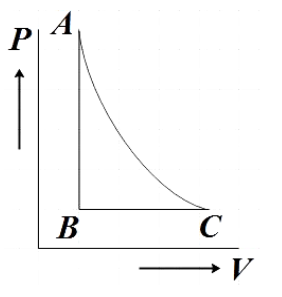
C.
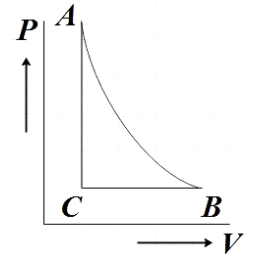
D.




Answer
574.8k+ views
Hint: Analyze the given PT diagram well. Find out the processes represented by AB and BC in the diagram and AC is already given. Recall from your prior knowledge how these processes are depicted in a PV diagram. Check which among the given PV diagrams correctly corresponds to the given PT diagram.
Complete answer:
In the question, we are given a PT diagram of an ideal gas with three processes marked AB, BC and AC and then we are asked to find the corresponding PV diagram.
We are directly given the question that AC is the graph representing adiabatic processes. Now let us analyze the PT diagram further to understand what processes AB and BC represent.
From the given diagram, we see that, for AB the temperature is constant. We know that the process in which the temperature remains constant is the isothermal process. Therefore, AB represents an isothermal process.
Now for BC, the pressure is remaining constant which from our prior knowledge we know further represents an isobaric process. Now that we have understood all the processes represented in the PT diagram let us make the corresponding PV diagram using the reference diagram given below.
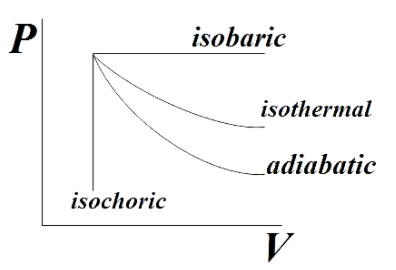
From the reference PV diagram we get that isobaric processes are drawn parallel to the Volume axis. So BC being an isobaric process will be parallel to the volume axis.
From the reference PV diagram we see that though the isothermal and adiabatic processes in a PV diagram seem similar, adiabatic process is steeper than that of isothermal process. So AC should be steeper than AB.
The PV diagram that satisfies all these condition is given by,
Hence, option B is the correct answer.
Note:
PV relation for isothermal process is given by,
$PV=k$
While the PV relation for adiabatic process is given by,
$P{{V}^{\gamma }}=k$
From these relations, we realize why adiabatic processes lie below the isothermal process. Though their PV diagrams appear similar both these processes are entirely opposite to each other in many ways.
Complete answer:
In the question, we are given a PT diagram of an ideal gas with three processes marked AB, BC and AC and then we are asked to find the corresponding PV diagram.
We are directly given the question that AC is the graph representing adiabatic processes. Now let us analyze the PT diagram further to understand what processes AB and BC represent.
From the given diagram, we see that, for AB the temperature is constant. We know that the process in which the temperature remains constant is the isothermal process. Therefore, AB represents an isothermal process.
Now for BC, the pressure is remaining constant which from our prior knowledge we know further represents an isobaric process. Now that we have understood all the processes represented in the PT diagram let us make the corresponding PV diagram using the reference diagram given below.

From the reference PV diagram we get that isobaric processes are drawn parallel to the Volume axis. So BC being an isobaric process will be parallel to the volume axis.
From the reference PV diagram we see that though the isothermal and adiabatic processes in a PV diagram seem similar, adiabatic process is steeper than that of isothermal process. So AC should be steeper than AB.
The PV diagram that satisfies all these condition is given by,

Hence, option B is the correct answer.
Note:
PV relation for isothermal process is given by,
$PV=k$
While the PV relation for adiabatic process is given by,
$P{{V}^{\gamma }}=k$
From these relations, we realize why adiabatic processes lie below the isothermal process. Though their PV diagrams appear similar both these processes are entirely opposite to each other in many ways.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




