
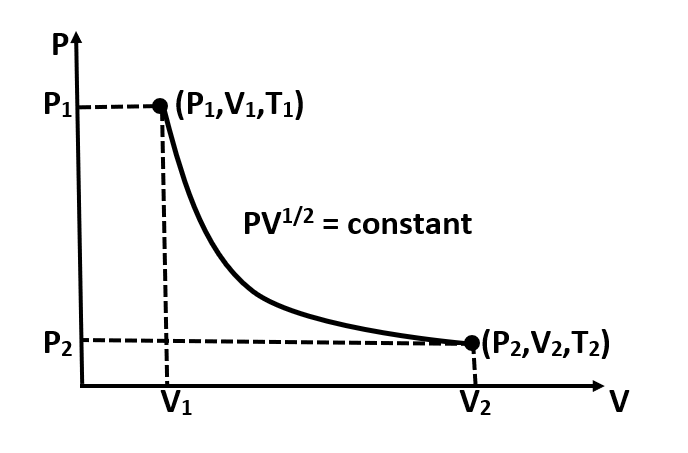
The P-V diagram of path followed by one mole of perfect gas in a cylindrical container is shown in figure, the work done when the gas is taken from state A to state B is:
$\begin{align}
& (A)2{{P}_{2}}{{V}_{1}}\left[ 1-\sqrt{\dfrac{{{V}_{2}}}{{{V}_{1}}}} \right] \\
& (B)2{{P}_{1}}{{V}_{1}}\left[ 1-\sqrt{\dfrac{{{V}_{1}}}{{{V}_{2}}}} \right] \\
& (C)2{{P}_{2}}{{V}_{2}}\left[ 1-\sqrt{\dfrac{{{V}_{1}}}{{{V}_{2}}}} \right] \\
& (D)2{{P}_{1}}{{V}_{2}}\left[ 1-\sqrt{\dfrac{{{V}_{1}}}{{{V}_{2}}}} \right] \\
\end{align}$

Answer
527.1k+ views
Hint: We have been given the condition that, $P{{V}^{\dfrac{1}{2}}}=k$ ,where k is any arbitrary constant. Now, this is an example of a polytropic process. So we will use the formula of work done in taking a gas from initial state to a final state of a polytropic process.
Complete answer:
It has been given in the question:
The initial parameters of gas are denoted by the following terms:
Initial volume : ${{V}_{1}}$
Initial pressure: ${{P}_{1}}$
Initial temperature: ${{T}_{1}}$
And, the final parameters of the gas are given by the following terms:
Final volume : ${{V}_{2}}$
Final pressure : ${{P}_{2}}$
Final temperature: ${{T}_{2}}$
Now, once we have defined the initial and final parameters of the gas, we can proceed ahead with calculating the work done in the given process.
As it is given:
$\Rightarrow P{{V}^{\dfrac{1}{2}}}=k$
This is a Polytropic process, with the value of :
$\Rightarrow n=\dfrac{1}{2}$
Now, the formula for work done in a polytropic process is given by the following equation:
$\Rightarrow W=\dfrac{{{P}_{1}}{{V}_{1}}-{{P}_{2}}{{V}_{2}}}{n-1}$
Putting the value of (n) in the above equation, we get:
$\begin{align}
& \Rightarrow W=\dfrac{{{P}_{1}}{{V}_{1}}-{{P}_{2}}{{V}_{2}}}{\dfrac{1}{2}-1} \\
& \Rightarrow W=2\left( {{P}_{2}}{{V}_{2}}-{{P}_{1}}{{V}_{1}} \right) \\
\end{align}$
Taking ${{P}_{2}}{{V}_{2}}$common out of the bracket in the right-hand side of the equation, we get the work done as:
$\Rightarrow W=2{{P}_{2}}{{V}_{2}}\left( 1-\dfrac{{{P}_{1}}{{V}_{1}}}{{{P}_{2}}{{V}_{2}}} \right)$ [Let this expression be equation number (1)]
Now, using the given polytropic function, we can write:
$\begin{align}
& \Rightarrow {{P}_{1}}{{({{V}_{1}})}^{\dfrac{1}{2}}}=k \\
& \Rightarrow {{P}_{1}}{{({{V}_{1}})}^{\dfrac{1}{2}}}\times {{(V)}^{\dfrac{1}{2}}}=k{{(V)}^{\dfrac{1}{2}}} \\
& \Rightarrow {{P}_{1}}{{V}_{1}}=k{{({{V}_{1}})}^{\dfrac{1}{2}}} \\
\end{align}$
Similarly, for the final state of gas we can write:
$\Rightarrow {{P}_{2}}{{V}_{2}}=k{{({{V}_{2}})}^{\dfrac{1}{2}}}$
Using these two equations in equation number (1), we get:
$\begin{align}
& \Rightarrow W=2{{P}_{2}}{{V}_{2}}\left( 1-\dfrac{k\sqrt{{{V}_{1}}}}{k\sqrt{{{V}_{2}}}} \right) \\
& \therefore W=2{{P}_{2}}{{V}_{2}}\left( 1-\dfrac{\sqrt{{{V}_{1}}}}{\sqrt{{{V}_{2}}}} \right) \\
\end{align}$
Hence, the work done in taking gas from $1\to 2$ under the given polytropic process is $2{{P}_{2}}{{V}_{2}}\left( 1-\sqrt{\dfrac{{{V}_{1}}}{{{V}_{2}}}} \right)$ .
So, the correct answer is “Option C”.
Note: In a polytropic process, (n) can take any value. If we keep on varying (n) we will see that for different values of (n), we get different processes. For example: for, $n=0$ , the process will become isobaric. For, $n=1$, the process will become isothermal, etc. Thus all the basic processes are a subset of the polytropic process.
Complete answer:
It has been given in the question:
The initial parameters of gas are denoted by the following terms:
Initial volume : ${{V}_{1}}$
Initial pressure: ${{P}_{1}}$
Initial temperature: ${{T}_{1}}$
And, the final parameters of the gas are given by the following terms:
Final volume : ${{V}_{2}}$
Final pressure : ${{P}_{2}}$
Final temperature: ${{T}_{2}}$
Now, once we have defined the initial and final parameters of the gas, we can proceed ahead with calculating the work done in the given process.
As it is given:
$\Rightarrow P{{V}^{\dfrac{1}{2}}}=k$
This is a Polytropic process, with the value of :
$\Rightarrow n=\dfrac{1}{2}$
Now, the formula for work done in a polytropic process is given by the following equation:
$\Rightarrow W=\dfrac{{{P}_{1}}{{V}_{1}}-{{P}_{2}}{{V}_{2}}}{n-1}$
Putting the value of (n) in the above equation, we get:
$\begin{align}
& \Rightarrow W=\dfrac{{{P}_{1}}{{V}_{1}}-{{P}_{2}}{{V}_{2}}}{\dfrac{1}{2}-1} \\
& \Rightarrow W=2\left( {{P}_{2}}{{V}_{2}}-{{P}_{1}}{{V}_{1}} \right) \\
\end{align}$
Taking ${{P}_{2}}{{V}_{2}}$common out of the bracket in the right-hand side of the equation, we get the work done as:
$\Rightarrow W=2{{P}_{2}}{{V}_{2}}\left( 1-\dfrac{{{P}_{1}}{{V}_{1}}}{{{P}_{2}}{{V}_{2}}} \right)$ [Let this expression be equation number (1)]
Now, using the given polytropic function, we can write:
$\begin{align}
& \Rightarrow {{P}_{1}}{{({{V}_{1}})}^{\dfrac{1}{2}}}=k \\
& \Rightarrow {{P}_{1}}{{({{V}_{1}})}^{\dfrac{1}{2}}}\times {{(V)}^{\dfrac{1}{2}}}=k{{(V)}^{\dfrac{1}{2}}} \\
& \Rightarrow {{P}_{1}}{{V}_{1}}=k{{({{V}_{1}})}^{\dfrac{1}{2}}} \\
\end{align}$
Similarly, for the final state of gas we can write:
$\Rightarrow {{P}_{2}}{{V}_{2}}=k{{({{V}_{2}})}^{\dfrac{1}{2}}}$
Using these two equations in equation number (1), we get:
$\begin{align}
& \Rightarrow W=2{{P}_{2}}{{V}_{2}}\left( 1-\dfrac{k\sqrt{{{V}_{1}}}}{k\sqrt{{{V}_{2}}}} \right) \\
& \therefore W=2{{P}_{2}}{{V}_{2}}\left( 1-\dfrac{\sqrt{{{V}_{1}}}}{\sqrt{{{V}_{2}}}} \right) \\
\end{align}$
Hence, the work done in taking gas from $1\to 2$ under the given polytropic process is $2{{P}_{2}}{{V}_{2}}\left( 1-\sqrt{\dfrac{{{V}_{1}}}{{{V}_{2}}}} \right)$ .
So, the correct answer is “Option C”.
Note: In a polytropic process, (n) can take any value. If we keep on varying (n) we will see that for different values of (n), we get different processes. For example: for, $n=0$ , the process will become isobaric. For, $n=1$, the process will become isothermal, etc. Thus all the basic processes are a subset of the polytropic process.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




