
The rate of diffusion of liquids is ________.
A) Higher than gases.
B) Lower than solids.
C) Higher than solids.
D) Equal to gases.
Answer
575.4k+ views
Hint: Diffusion is the process of movement of net free movement of molecules from higher concentration to lower concentration. It depends on temperature and the gradient concentration.
Complete answer:
> We know that matter is composed of molecules and particles at microscopic level. Since the rate of diffusion depends on the free movement of particles and molecules hence we need to study the arrangement of particles in matter and according to the arrangement of particles matter is divided into three subcategories of: solid, liquid and gas.
> Solids have definite shape and volume, that is there is no intermolecular space between its molecules. Its molecules are tightly bound with each other under strong force of attraction. Hence the movement of its molecules is restricted.
> Liquids have definite volume but not definite shape. There is intermolecular space between the molecules of the liquid. Liquid has both viscosity and fluidity. Molecules in liquid can move freely in any direction.
> Gases have neither definite volume nor definite shape it has a lot of intermolecular space and the molecules are far apart from each other making the movement free and easy without touching its other constituent molecules.
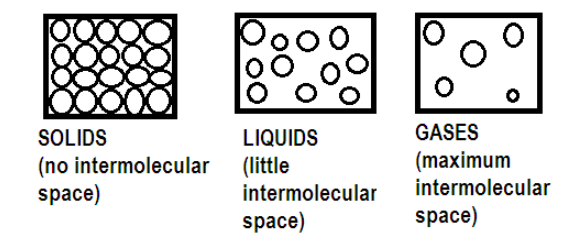
Hence we can conclude the rate of diffusion of liquids is higher than solids due to the free movement of molecules and lack of strong force of attraction in liquids but rate of diffusion of liquids is lower than gases as in gases molecules are quite far apart.
Hence the correct option is option C.
Note: Osmosis is also the special type of diffusion where the movement of molecules occur across a membrane due to change in concentration .
Complete answer:
> We know that matter is composed of molecules and particles at microscopic level. Since the rate of diffusion depends on the free movement of particles and molecules hence we need to study the arrangement of particles in matter and according to the arrangement of particles matter is divided into three subcategories of: solid, liquid and gas.
> Solids have definite shape and volume, that is there is no intermolecular space between its molecules. Its molecules are tightly bound with each other under strong force of attraction. Hence the movement of its molecules is restricted.
> Liquids have definite volume but not definite shape. There is intermolecular space between the molecules of the liquid. Liquid has both viscosity and fluidity. Molecules in liquid can move freely in any direction.
> Gases have neither definite volume nor definite shape it has a lot of intermolecular space and the molecules are far apart from each other making the movement free and easy without touching its other constituent molecules.

Hence we can conclude the rate of diffusion of liquids is higher than solids due to the free movement of molecules and lack of strong force of attraction in liquids but rate of diffusion of liquids is lower than gases as in gases molecules are quite far apart.
Hence the correct option is option C.
Note: Osmosis is also the special type of diffusion where the movement of molecules occur across a membrane due to change in concentration .
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
Differentiate between an exothermic and an endothermic class 11 chemistry CBSE

10 examples of friction in our daily life

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Difference Between Prokaryotic Cells and Eukaryotic Cells

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




