
The reaction which involves dichlorocarbene as an electrophile is:
A) Reimer-Tiemann reaction
B) Kolbe’s reaction
C) Friedel-Crafts acylation
D) Fittings reaction
Answer
577.8k+ views
Hint: In electrophilic substitution reactions the dichlorocarbene acts as an electrophile. The negative charge on the ring attacks on the dichlorocarbene. This results in the substitution of protons of the molecule by the dichlorocarbene.
Complete answer:
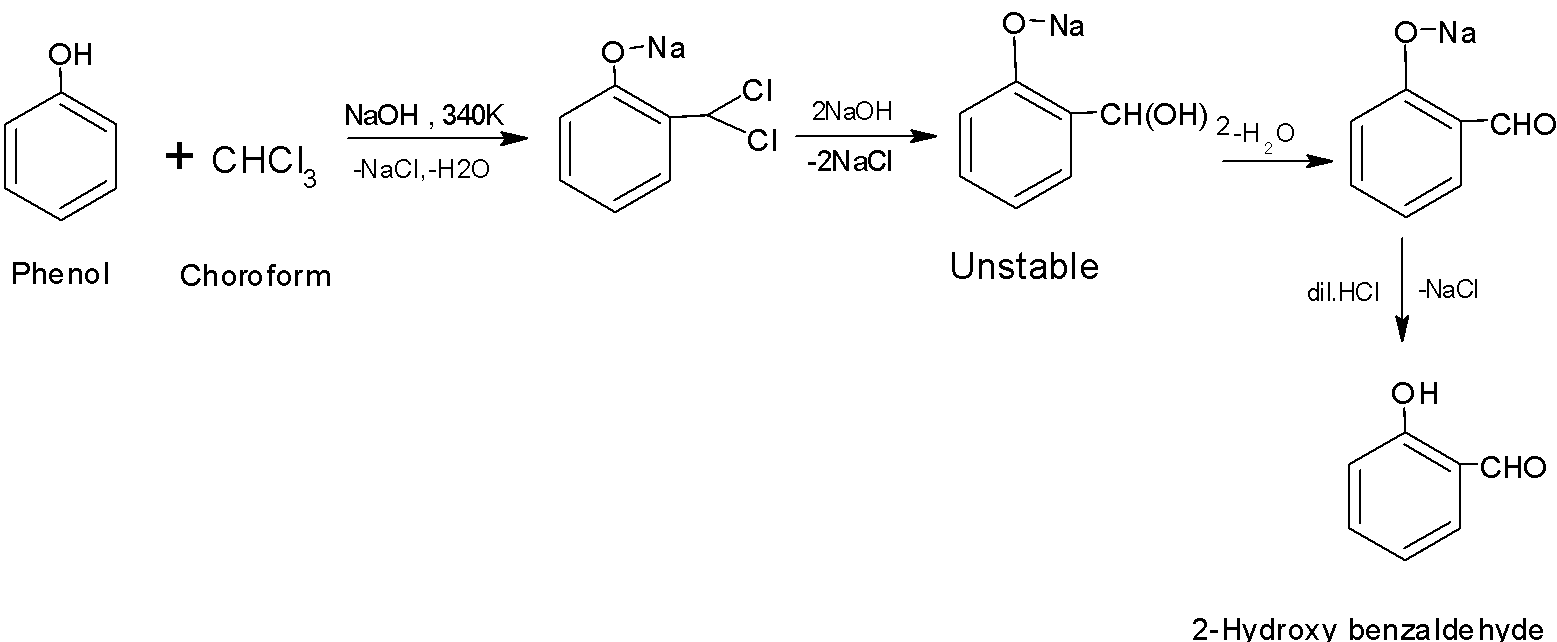
When phenol is refluxed with the chloroform in the presence of an aqueous solution of sodium hydroxide at \[\text{340K}\] followed by the hydrolysis, the aldehydic group \[\text{(-CH=O)}\]gets introduced in the ring at the ortho position to the phenolic group. Ortho hydroxybenzaldehyde is formed as the product of the reaction. This reaction of the introduction of the aldehyde group on the phenol is called the Reimer-Tiemann reaction.
For example, Phenol undergoes the Reimer -Tiemann reaction to form salicylaldehyde.
Now let's have a look at the mechanism of reaction:
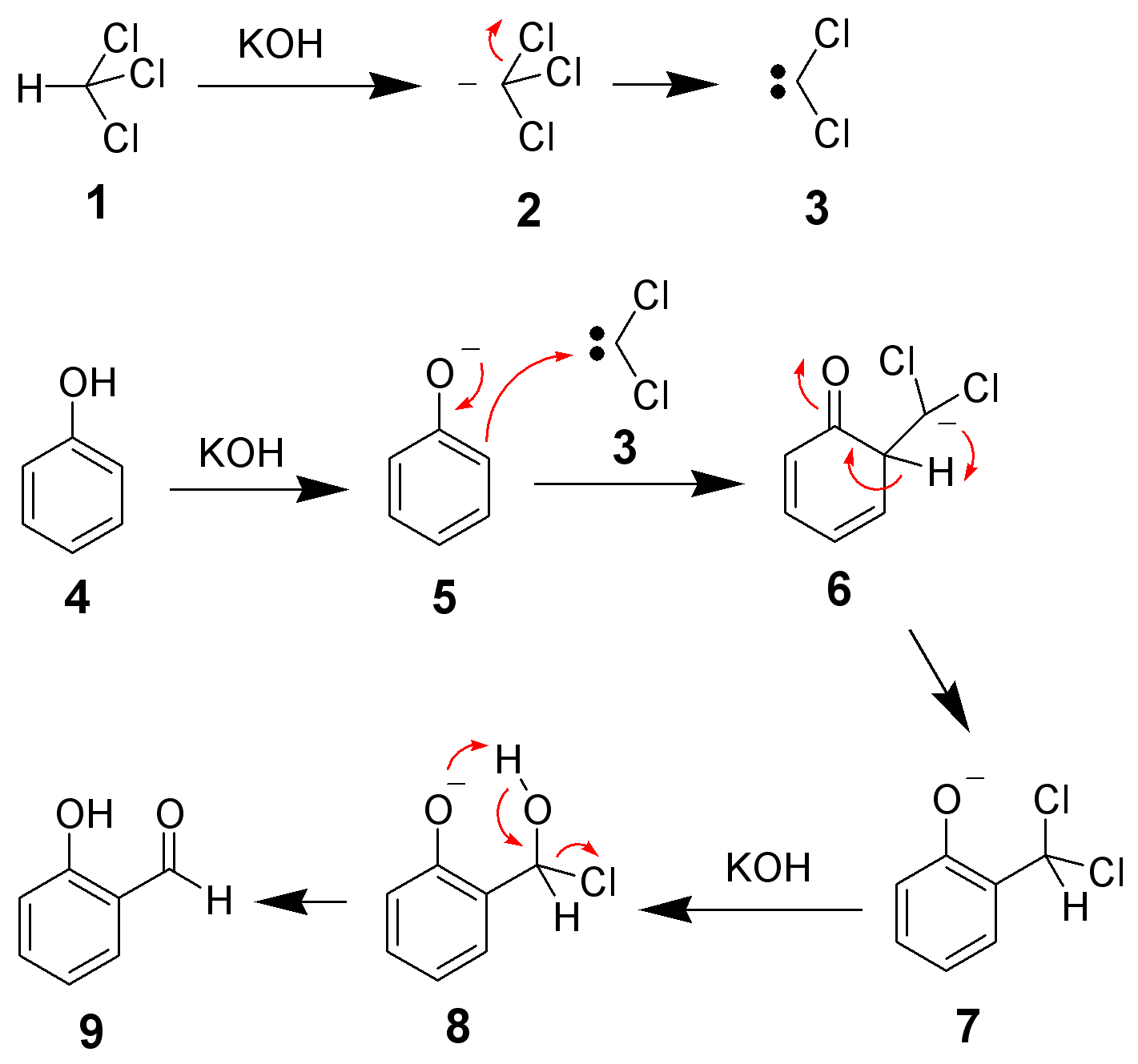
The Reimer-Tiemann reaction is an electrophilic substitution reaction. In the Reimer Tiemann reaction, the dichlorocarbene acts as the electrophile.
In the first step of the reaction, the dichlorocarbene is generated by the reaction of chloroform $\text{CHC}{{\text{l}}_{\text{3}}}$with the \[\text{NaOH}\]as:
$\begin{align}
& \text{CHC}{{\text{l}}_{\text{3}}}\text{ + O}{{\text{H}}^{\text{-}}}\text{ }\to \text{ }{{\text{H}}_{\text{2}}}\text{O + CCl}_{\text{3}}^{\text{-}}\to \text{C}{{\text{l}}^{\text{-}}}\text{ + :CC}{{\text{l}}_{\text{2}}} \\
& \text{ (}Dichlorocaebene) \\
\end{align}$
Dichlorocarbene is a reactive and neutral species that contains the divalent carbon. The carbon holds the one lone pair of an electron which can exist in either singlet or triplet state. It acts as the electrophile. Dichlorocarbene contains the carbon atom with the sextet of electrons and thus readily attacks on positive canters. The electrophilic substitution reaction occurs as:
Dichlorocarbene attacks on the phenol.
Thus Reimer -Tieman reactions involve the dichlorocarbene as the electrophile.
Let's have a look at other reactions.
The reaction of sodium phenoxide when heated with carbon dioxide at about \[\text{400K}\] and under the pressure of 4 to 7 atm to form a sodium salicylate as the major product is called the Kolbe’s reaction. In Kolbe’s reaction, \[\text{C}{{\text{O}}_{\text{2}}}\]acts as the electrophile. The mechanism involves the attack of\[\text{C}{{\text{O}}_{\text{2}}}\] on phenoxide ion.
In Friedel craft acylation and fitting reaction, dichlorocarbene is not involved.
Thus, the Reimer-Tiemann reaction uses the dichlorocarbene as the electrophile. The remaining reactions use different electrophiles for the conversion of reactants into the product.
Hence, (A) is the correct option.
Additional information:
A carbene is a neutral reactive species. It acts as the intermediate.
The carbon has four electrons in its valence shell out of which two remain unshared to give the carbene. The general structure of carbene is as:
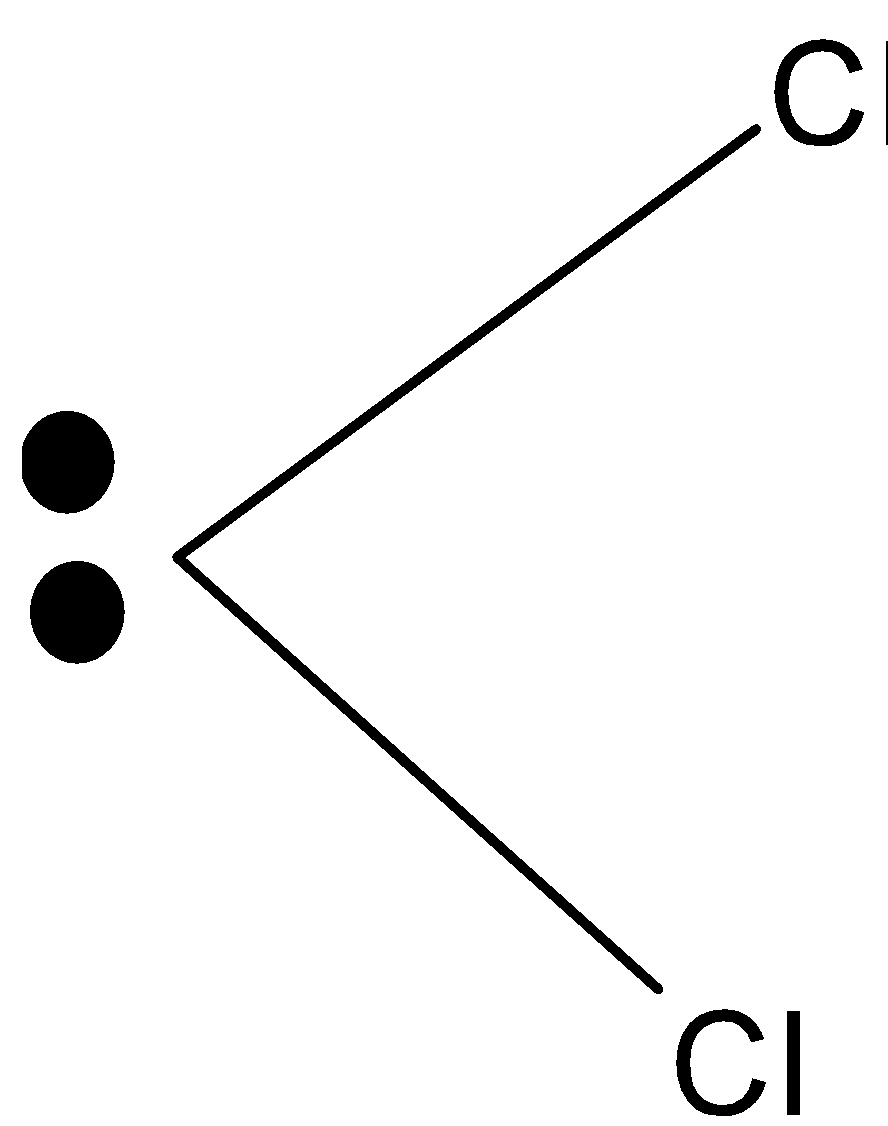
\[\text{C}{{\text{H}}_{\text{2}}}\]Is the parent carbene from which other carbenes are derived.
In carbene, the carbon atom has two unshared electrons in the p-orbitals. According to the spins of the unshared electron, a carbene is classified as a singlet and triplet.
In the singlet state, the carbon is in the \[s{{p}^{2}}\] hybridization state. One of the orbits \[s{{p}^{2}}\] contains the unshared pair of electrons such that both electrons have opposite spin. It is diamagnetic.
In most of the triplet carbene, the carbon atom is $sp$ hybridized. Such that each p-orbitals host an electron. The two-electron have a parallel spin occupied in \[{{\text{p}}_{\text{y}}}\] and \[{{\text{p}}_{\text{z}}}\]orbitals. They are linear. Such carbene is paramagnetic.
Note:
In electrophile is an electron loving species. The negative charge attacks on the carbene such that it replaces the hydrogen on the ring. Carbon tetrachloride can also be used to generate a dichlorocarbene. Always remember that in the organic mechanism, the electron-rich species attacks on the electron-deficient species, and arrows depict the movement of electrons.
Complete answer:
When phenol is refluxed with the chloroform in the presence of an aqueous solution of sodium hydroxide at \[\text{340K}\] followed by the hydrolysis, the aldehydic group \[\text{(-CH=O)}\]gets introduced in the ring at the ortho position to the phenolic group. Ortho hydroxybenzaldehyde is formed as the product of the reaction. This reaction of the introduction of the aldehyde group on the phenol is called the Reimer-Tiemann reaction.
For example, Phenol undergoes the Reimer -Tiemann reaction to form salicylaldehyde.

Now let's have a look at the mechanism of reaction:
The Reimer-Tiemann reaction is an electrophilic substitution reaction. In the Reimer Tiemann reaction, the dichlorocarbene acts as the electrophile.
In the first step of the reaction, the dichlorocarbene is generated by the reaction of chloroform $\text{CHC}{{\text{l}}_{\text{3}}}$with the \[\text{NaOH}\]as:
$\begin{align}
& \text{CHC}{{\text{l}}_{\text{3}}}\text{ + O}{{\text{H}}^{\text{-}}}\text{ }\to \text{ }{{\text{H}}_{\text{2}}}\text{O + CCl}_{\text{3}}^{\text{-}}\to \text{C}{{\text{l}}^{\text{-}}}\text{ + :CC}{{\text{l}}_{\text{2}}} \\
& \text{ (}Dichlorocaebene) \\
\end{align}$
Dichlorocarbene is a reactive and neutral species that contains the divalent carbon. The carbon holds the one lone pair of an electron which can exist in either singlet or triplet state. It acts as the electrophile. Dichlorocarbene contains the carbon atom with the sextet of electrons and thus readily attacks on positive canters. The electrophilic substitution reaction occurs as:
Dichlorocarbene attacks on the phenol.

Thus Reimer -Tieman reactions involve the dichlorocarbene as the electrophile.
Let's have a look at other reactions.
The reaction of sodium phenoxide when heated with carbon dioxide at about \[\text{400K}\] and under the pressure of 4 to 7 atm to form a sodium salicylate as the major product is called the Kolbe’s reaction. In Kolbe’s reaction, \[\text{C}{{\text{O}}_{\text{2}}}\]acts as the electrophile. The mechanism involves the attack of\[\text{C}{{\text{O}}_{\text{2}}}\] on phenoxide ion.
In Friedel craft acylation and fitting reaction, dichlorocarbene is not involved.
Thus, the Reimer-Tiemann reaction uses the dichlorocarbene as the electrophile. The remaining reactions use different electrophiles for the conversion of reactants into the product.
Hence, (A) is the correct option.
Additional information:
A carbene is a neutral reactive species. It acts as the intermediate.
The carbon has four electrons in its valence shell out of which two remain unshared to give the carbene. The general structure of carbene is as:

\[\text{C}{{\text{H}}_{\text{2}}}\]Is the parent carbene from which other carbenes are derived.
In carbene, the carbon atom has two unshared electrons in the p-orbitals. According to the spins of the unshared electron, a carbene is classified as a singlet and triplet.
In the singlet state, the carbon is in the \[s{{p}^{2}}\] hybridization state. One of the orbits \[s{{p}^{2}}\] contains the unshared pair of electrons such that both electrons have opposite spin. It is diamagnetic.
In most of the triplet carbene, the carbon atom is $sp$ hybridized. Such that each p-orbitals host an electron. The two-electron have a parallel spin occupied in \[{{\text{p}}_{\text{y}}}\] and \[{{\text{p}}_{\text{z}}}\]orbitals. They are linear. Such carbene is paramagnetic.
Note:
In electrophile is an electron loving species. The negative charge attacks on the carbene such that it replaces the hydrogen on the ring. Carbon tetrachloride can also be used to generate a dichlorocarbene. Always remember that in the organic mechanism, the electron-rich species attacks on the electron-deficient species, and arrows depict the movement of electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




