
The reagent that can distinguish between 1-hexyne and 1-hexene is:
A. \[Ag{\left( {N{H_3}} \right)_2}^ + \]
B. \[KMn{O_4}\]
C. \[B{r_2}inCC{l_4}\]
D. \[{H_2}S{O_4}\]
Answer
574.2k+ views
Hint:To distinguish one from another use reactions where change of physical characteristics is happening. For example, change of colour of the solution, precipitation takes place, generation of effervescence etc. Structures of 1-hexyne and 1-hexene are
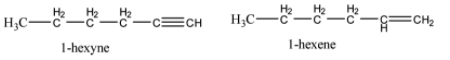
Complete step by step answer:
When both are treated with \[KMn{O_4}\] , oxidation of alkyne and alkene is happened as well as decoloration of \[{\text{KMn}}{{\text{O}}_4}\] is happened. Which is shown below.
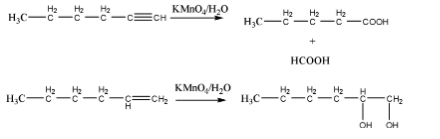
So, this is not an appropriate reagent to distinguish between 1-hexyne and 1-hexene.
When both are treated with \[B{r_2}inCC{l_4}\] addition reaction takes place to the double bond and triple bond. Asa result, decoloration of bromine takes place. Which is shown below.
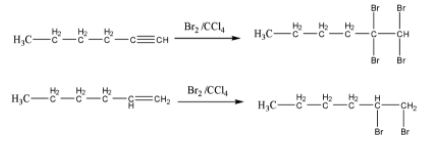
So, this is not an appropriate reagent to distinguish between 1-hexyne and 1-hexene.
When both are treated with \[{H_2}S{O_4}\] hydration of alkyne and alkene takes place, and corresponding ketone and alcohol is formed respectively. Which is shown below,
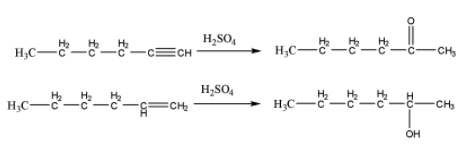
So, this is not an appropriate reagent to distinguish between 1-hexyne and 1-hexene.
When both are treated with \[Ag{\left( {N{H_3}} \right)_2}^ + \] which is called tollen’s reagent, the terminal alkyne reacts with tollen’s reagent and gives white precipitate of acetylide. On the other hand, alkene does not react with it. These reactions are shown below,
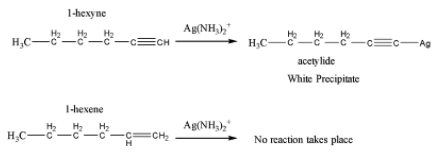
From this reaction a change of physical characteristic happens which can be visualized to understand the difference between the 1-hexyne and 1-hexene.
So, the correct option is A.
Note:
Remember that tollen’s reagent reacts with only terminal alkyne compounds not with all alkynes. It also can be used to distinguish between terminal alkyne and non-terminal alkyne. Reagent can be a substance or mixture of compounds which are used in chemical analysis or reactions. When a reagent is added to a system it causes chemical reactions. For a particular reaction, a particular reagent gets consumed in the process of the chemical reaction.
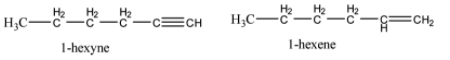
Complete step by step answer:
When both are treated with \[KMn{O_4}\] , oxidation of alkyne and alkene is happened as well as decoloration of \[{\text{KMn}}{{\text{O}}_4}\] is happened. Which is shown below.

So, this is not an appropriate reagent to distinguish between 1-hexyne and 1-hexene.
When both are treated with \[B{r_2}inCC{l_4}\] addition reaction takes place to the double bond and triple bond. Asa result, decoloration of bromine takes place. Which is shown below.

So, this is not an appropriate reagent to distinguish between 1-hexyne and 1-hexene.
When both are treated with \[{H_2}S{O_4}\] hydration of alkyne and alkene takes place, and corresponding ketone and alcohol is formed respectively. Which is shown below,

So, this is not an appropriate reagent to distinguish between 1-hexyne and 1-hexene.
When both are treated with \[Ag{\left( {N{H_3}} \right)_2}^ + \] which is called tollen’s reagent, the terminal alkyne reacts with tollen’s reagent and gives white precipitate of acetylide. On the other hand, alkene does not react with it. These reactions are shown below,

From this reaction a change of physical characteristic happens which can be visualized to understand the difference between the 1-hexyne and 1-hexene.
So, the correct option is A.
Note:
Remember that tollen’s reagent reacts with only terminal alkyne compounds not with all alkynes. It also can be used to distinguish between terminal alkyne and non-terminal alkyne. Reagent can be a substance or mixture of compounds which are used in chemical analysis or reactions. When a reagent is added to a system it causes chemical reactions. For a particular reaction, a particular reagent gets consumed in the process of the chemical reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




