
The reagent(s) used for converting ethanoic acid to ethanol is/are:
(A) $LiAl{H_4}$
(B) $B{H_3}$ in THF
(C) $PC{l_3}$
(D) ${K_2}C{r_2}{O_7}/{H^ + }$
Answer
584.1k+ views
Hint: The reaction of obtaining ethanol from ethanoic acid is a reduction reaction. A hydride donor can give this reaction. A suitable reducing agent is required to obtain this conversion.
Complete step by step solution:
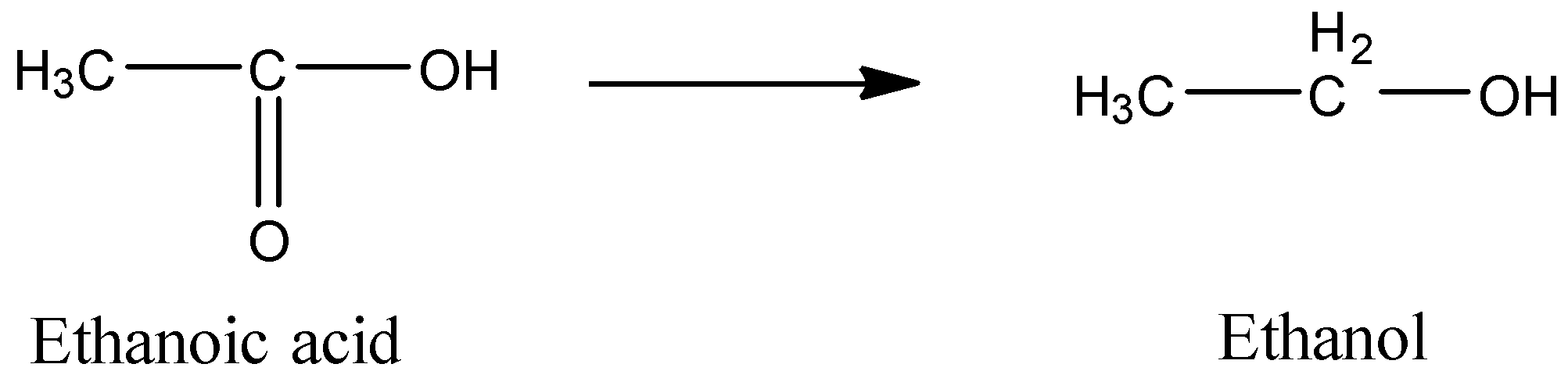
Let’s see the structures of ethanoic acid and ethanol to have a better idea about the reagents.
We will have a look at all the given reagents in order to see if they would give this conversion or not.
A) $LiAl{H_4}$
- It is called lithium aluminum hydride. As the name suggests, it has hydride and it can reduce the double or triple bonds of carbon. $LiAl{H_4}$ is a strong reducing agent and easily reduces the carboxylic acid group into an alcohol functional group. So, this reagent can give this conversion.
B) $B{H_3}{\text{ in THF}}$
- $B{H_3}$ is known as borane and THF is a solvent and stands for tetrahydrofuran.
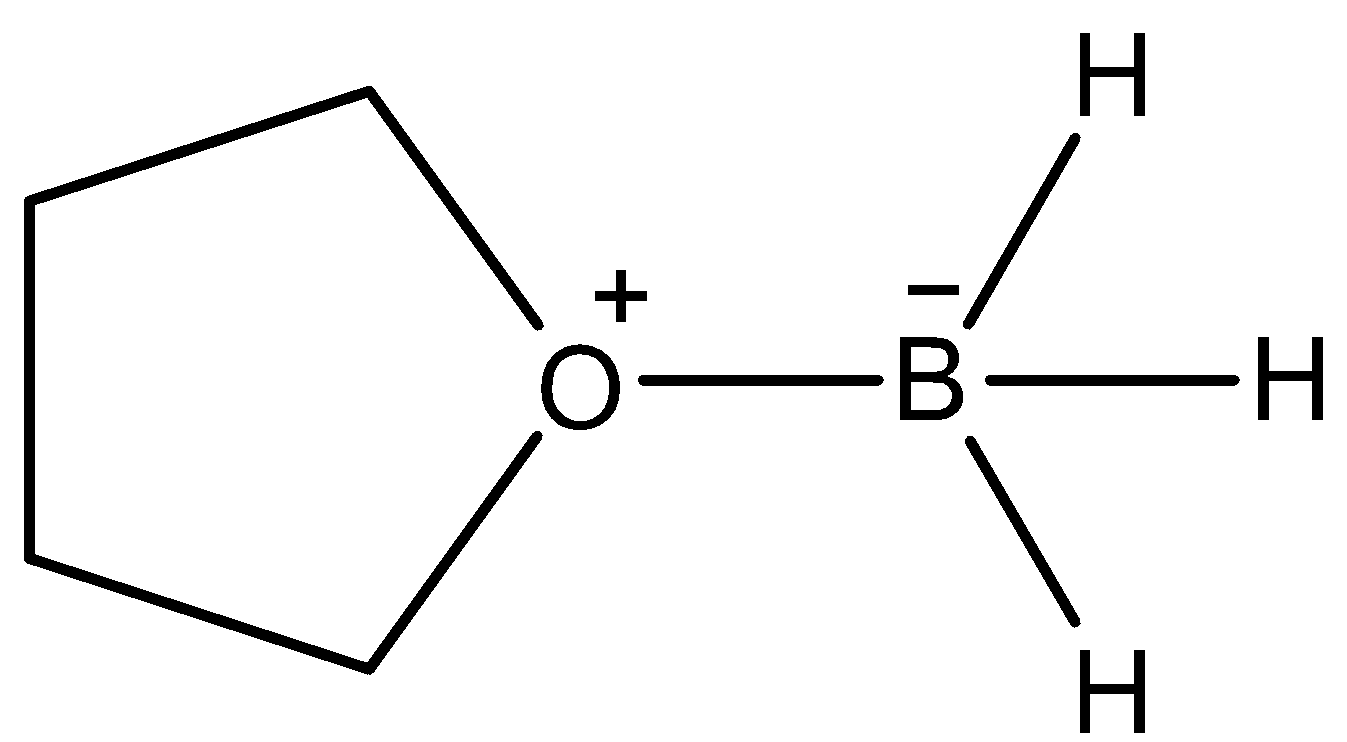
- This reagent not only does hydroboration across the carbon-carbon double bond but also reduces some functional groups. It can reduce the carboxylic acid functional group to corresponding alcohols. This reagent is supposed to give this conversion due to the formation of the following complex.
C) $PC{l_3}$
- Phosphorus trichloride is a chlorinating agent and can add –Cl group in place of the hydroxyl functional group. Thus, it is useful in the preparation of chloro derivatives. So, it cannot give ethanol from ethanoic acid.
D) ${K_2}C{r_2}{O_7}/{H^ + }$
- Potassium dichromate produces chromic acid on reaction with acid. This reagent is a strong oxidising agent. So, this reagent is used for the oxidation of the compounds. So, it cannot be used for conversion of ethanoic acid to ethanol.
So, we can conclude that both $LiAl{H_4}$ and $B{H_3}/THF$ are used for the given conversion.
Therefore, correct answers are (A) and (B).
Note: Note that alongside lithium aluminum hydride and borane, sodium borohydride ($NaB{H_4}$) can also give this conversion. Ethanoic acid is also known as acetic acid as there are a total of two carbons present in the compound.
Complete step by step solution:
Let’s see the structures of ethanoic acid and ethanol to have a better idea about the reagents.

We will have a look at all the given reagents in order to see if they would give this conversion or not.
A) $LiAl{H_4}$
- It is called lithium aluminum hydride. As the name suggests, it has hydride and it can reduce the double or triple bonds of carbon. $LiAl{H_4}$ is a strong reducing agent and easily reduces the carboxylic acid group into an alcohol functional group. So, this reagent can give this conversion.
B) $B{H_3}{\text{ in THF}}$
- $B{H_3}$ is known as borane and THF is a solvent and stands for tetrahydrofuran.
- This reagent not only does hydroboration across the carbon-carbon double bond but also reduces some functional groups. It can reduce the carboxylic acid functional group to corresponding alcohols. This reagent is supposed to give this conversion due to the formation of the following complex.

C) $PC{l_3}$
- Phosphorus trichloride is a chlorinating agent and can add –Cl group in place of the hydroxyl functional group. Thus, it is useful in the preparation of chloro derivatives. So, it cannot give ethanol from ethanoic acid.
D) ${K_2}C{r_2}{O_7}/{H^ + }$
- Potassium dichromate produces chromic acid on reaction with acid. This reagent is a strong oxidising agent. So, this reagent is used for the oxidation of the compounds. So, it cannot be used for conversion of ethanoic acid to ethanol.
So, we can conclude that both $LiAl{H_4}$ and $B{H_3}/THF$ are used for the given conversion.
Therefore, correct answers are (A) and (B).
Note: Note that alongside lithium aluminum hydride and borane, sodium borohydride ($NaB{H_4}$) can also give this conversion. Ethanoic acid is also known as acetic acid as there are a total of two carbons present in the compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




