
The shape of \[3p\] orbital resembles ________.
Answer
502.8k+ views
Hint: We need to know that the atom is the smallest particle which is found in a chemical element. Each solid, liquid, and gas are composed with ionized or neutral atoms. An atom is made up of three subatomic particles and that is, neutrons, protons and electrons. And the atom containing the orbital and the electrons are located on this orbital. The orbitals are moved around the atom with electrons. And the orbitals are in four types, which is s – orbital, p – orbital, d – orbital and f – orbital.
Complete answer:
They express the wave behavior of an electron, a pair of electrons or nucleons. An orbital consists of two electrons which have paired spins. There are a total four orbitals, s, p, d and f and each orbital has different shapes and the properties. The shape of \[3p\] orbital favors the dumbbell – shape. Let’s see the shape of \[3p\]-orbital;
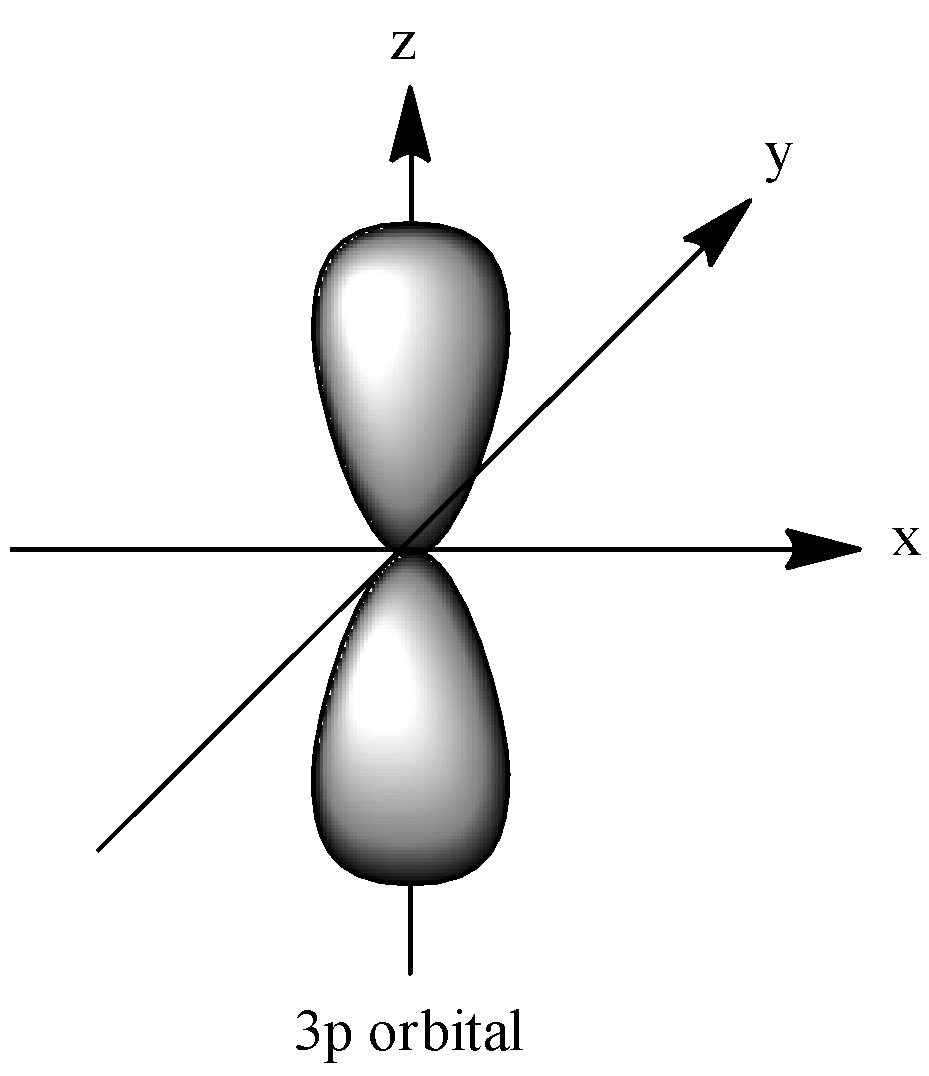
The p – orbital contains two sections which are known as lobes and it rests on either side of the plane moving through the nucleus. The \[3p\] orbitals are identical in terms of shape, size and the energy. The lobes present in the p – orbital lie along the x, y or the z – axis. And it can be denoted as, \[2{p_x},2{p_y},\] and \[2{p_z}\]. These three axes are mutually perpendicular. And when the principal quantum number increases, the energy and the size will also increase. Hence, the order is \[4p > 3p > 2p\].
Note:
We need to remember that the number of orbital present in the atom is four which is, s, p, d, and f. Each orbital is identical and it has a unique shape and different energy levels. The shape of p orbital is a dumb-bell shape structure. And the shape of the s-orbital is a spherical structure. The d-orbital has a clover shape and the f-orbital has different type’s structures.
Complete answer:
They express the wave behavior of an electron, a pair of electrons or nucleons. An orbital consists of two electrons which have paired spins. There are a total four orbitals, s, p, d and f and each orbital has different shapes and the properties. The shape of \[3p\] orbital favors the dumbbell – shape. Let’s see the shape of \[3p\]-orbital;

The p – orbital contains two sections which are known as lobes and it rests on either side of the plane moving through the nucleus. The \[3p\] orbitals are identical in terms of shape, size and the energy. The lobes present in the p – orbital lie along the x, y or the z – axis. And it can be denoted as, \[2{p_x},2{p_y},\] and \[2{p_z}\]. These three axes are mutually perpendicular. And when the principal quantum number increases, the energy and the size will also increase. Hence, the order is \[4p > 3p > 2p\].
Note:
We need to remember that the number of orbital present in the atom is four which is, s, p, d, and f. Each orbital is identical and it has a unique shape and different energy levels. The shape of p orbital is a dumb-bell shape structure. And the shape of the s-orbital is a spherical structure. The d-orbital has a clover shape and the f-orbital has different type’s structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




