
The shape of $BC{{l}_{3}}$, $PC{{l}_{3}}$, and $IC{{l}_{3}}$ molecules are:
(A) Triangular
(B) Pyramidal
(C) T-shaped
(D) All above are incorrect
Answer
534k+ views
Hint: The VSEPR model can be used to determine the shape of a molecule. The valence shell electron pair repulsion theory states that the molecules around the central atom will arrange in such a way that there is minimum repulsion between valence electron pairs of the atom.
Complete answer:
The VSEPR model of arrangement helps in increasing the stability of the molecule and decreasing its energy.
The repulsion between the electron pair increases in the following order
Bond pair-bond pair < bond pair-lone pair < lone pair-lone pair
Now, the formula $A{{X}_{n}}{{E}_{m}}$ can be used while applying the VSEPR theory to represent the number of electron pairs around a central atom.
Where the central atom is represented by A,
ligand bonded to the central atom is represented by X, and
lone pairs are represented by E.
Subscripts n and m represent the number of ligands and the number of lone pairs.
Steric Number = n + m
The sum of the coordination number of the central atom (number of atoms bonded to the central atom) and the number of valence electron lone pairs on the central atom gives the steric number of the compound.
Now, the molecular geometry of the compound according to the steric number and the $A{{X}_{n}}{{E}_{m}}$ formula are
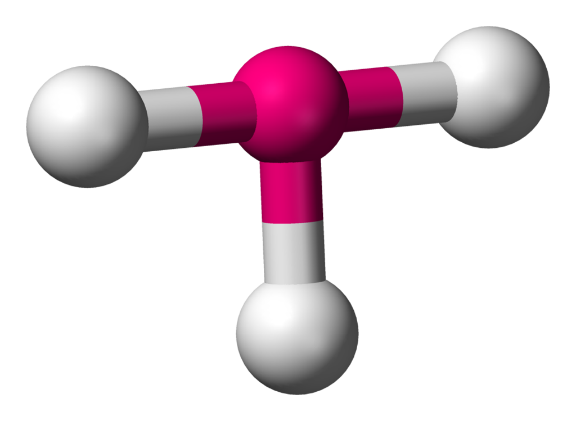
Now, in $BC{{l}_{3}}$, the valency of the central boron atom is 3. Since it is single-bonded with three chlorine atoms, there are no lone pairs.
So, the steric number is 3+0 = 3.
Hence it is of the molecule type $A{{X}_{3}}{{E}_{0}}$ and has a trigonal planar shape where all bond angles are $120{}^\circ $.
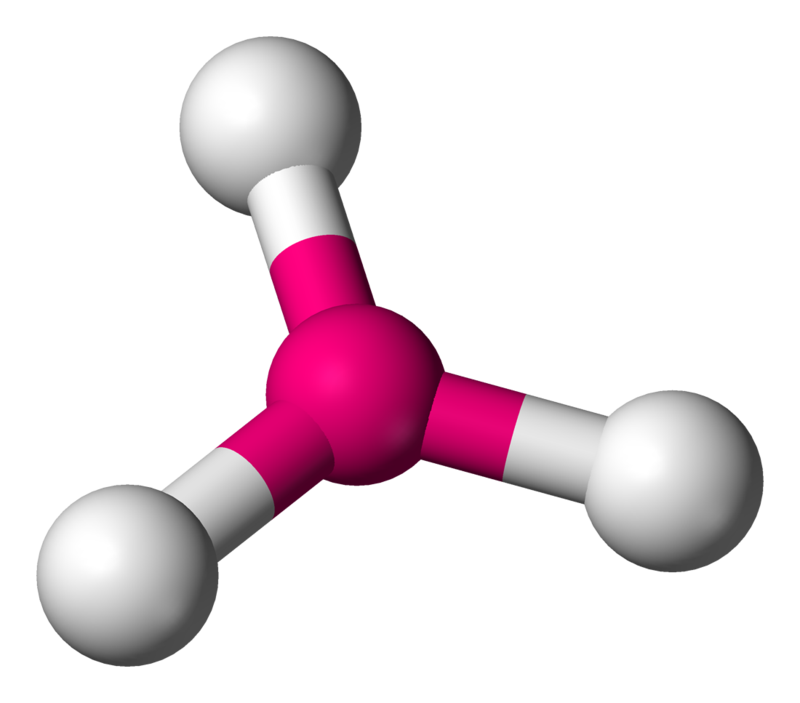
Now, in $PC{{l}_{3}}$, the valency of the central phosphorus atom is 3. Since it is single-bonded with three chlorine atoms, there are no lone pairs.
So, the steric number is 3+0=3.
Hence it is of the molecule type $A{{X}_{3}}{{E}_{0}}$ and has a trigonal planar shape where all bond angles are $120{}^\circ $.
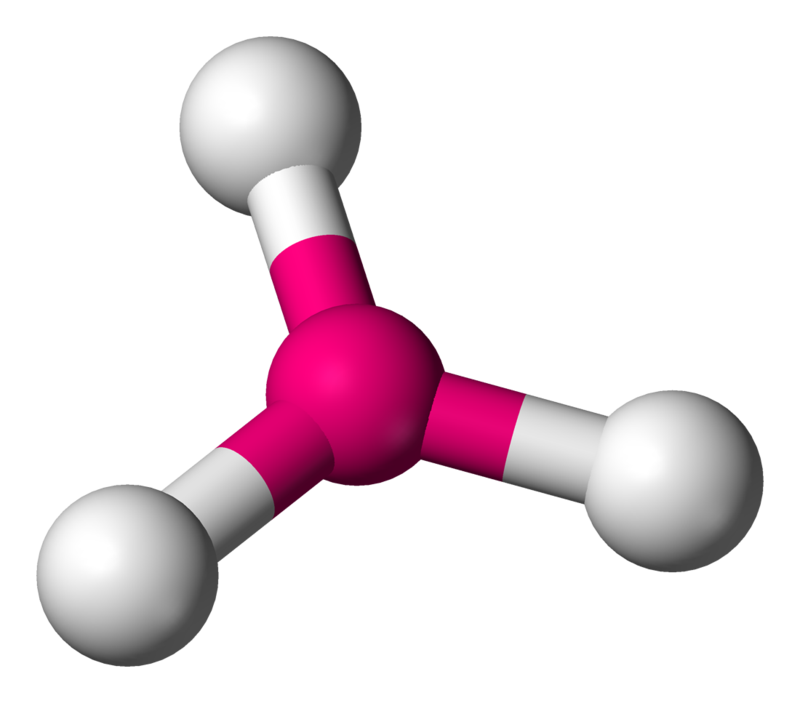
Now, in $IC{{l}_{3}}$, the valency of the central iodine atom is 7. Since it is single-bonded with three chlorine atoms, there are two lone pairs.
So, the steric number is 3+2=5.
Hence it is of the molecule type $A{{X}_{3}}{{E}_{2}}$ and has a t-shape where the bond angles are $90{}^\circ $ and $180{}^\circ $.
So, the answer to the question is option (D) all above are incorrect.
Note:
It should be noted that the VSEPR theory does not explain the molecular geometry and shapes of the isoelectronic species. It also does not properly explain the molecules of transition metals. It does not consider the inactive lone pairs and the size of the ligand attached to the central atom.
Complete answer:
The VSEPR model of arrangement helps in increasing the stability of the molecule and decreasing its energy.
The repulsion between the electron pair increases in the following order
Bond pair-bond pair < bond pair-lone pair < lone pair-lone pair
Now, the formula $A{{X}_{n}}{{E}_{m}}$ can be used while applying the VSEPR theory to represent the number of electron pairs around a central atom.
Where the central atom is represented by A,
ligand bonded to the central atom is represented by X, and
lone pairs are represented by E.
Subscripts n and m represent the number of ligands and the number of lone pairs.
Steric Number = n + m
The sum of the coordination number of the central atom (number of atoms bonded to the central atom) and the number of valence electron lone pairs on the central atom gives the steric number of the compound.
Now, the molecular geometry of the compound according to the steric number and the $A{{X}_{n}}{{E}_{m}}$ formula are
| STERIC NUMBER | $A{{X}_{n}}{{E}_{m}}$ | MOLECULAR GEOMETRY |
| 2 | $A{{X}_{2}}{{E}_{0}}$ | Linear |
| 3 | $A{{X}_{3}}{{E}_{0}}$ | Trigonal Planar |
| 3 | $A{{X}_{2}}{{E}_{1}}$ | Bent |
| 4 | $A{{X}_{4}}{{E}_{0}}$ | Tetrahedral |
| 4 | $A{{X}_{3}}{{E}_{1}}$ | Trigonal Pyramidal |
| 4 | $A{{X}_{2}}{{E}_{2}}$ | Bent |
| 5 | $A{{X}_{5}}{{E}_{0}}$ | Trigonal Bipyramidal |
| 5 | $A{{X}_{4}}{{E}_{1}}$ | Seesaw |
| 5 | $A{{X}_{3}}{{E}_{2}}$ | T-Shaped |
| 5 | $A{{X}_{2}}{{E}_{3}}$ | Linear |
Now, in $BC{{l}_{3}}$, the valency of the central boron atom is 3. Since it is single-bonded with three chlorine atoms, there are no lone pairs.
So, the steric number is 3+0 = 3.
Hence it is of the molecule type $A{{X}_{3}}{{E}_{0}}$ and has a trigonal planar shape where all bond angles are $120{}^\circ $.

Now, in $PC{{l}_{3}}$, the valency of the central phosphorus atom is 3. Since it is single-bonded with three chlorine atoms, there are no lone pairs.
So, the steric number is 3+0=3.
Hence it is of the molecule type $A{{X}_{3}}{{E}_{0}}$ and has a trigonal planar shape where all bond angles are $120{}^\circ $.

Now, in $IC{{l}_{3}}$, the valency of the central iodine atom is 7. Since it is single-bonded with three chlorine atoms, there are two lone pairs.
So, the steric number is 3+2=5.
Hence it is of the molecule type $A{{X}_{3}}{{E}_{2}}$ and has a t-shape where the bond angles are $90{}^\circ $ and $180{}^\circ $.

So, the answer to the question is option (D) all above are incorrect.
Note:
It should be noted that the VSEPR theory does not explain the molecular geometry and shapes of the isoelectronic species. It also does not properly explain the molecules of transition metals. It does not consider the inactive lone pairs and the size of the ligand attached to the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




