
“The shape of $BF_{3}$ molecule is trigonal planar.” State whether the given statement is true or false.
A. True
B. False
Answer
569.7k+ views
Hint: Shape and hybridization of a molecule can be calculated by using some rules i.e. first write the Lewis structure then calculate the number of sigma bonds and lone pair then determine the steric number (steric number is composed of number of lone pairs and sigma bonds) and then finally assign the hybridization and shape of the molecule.
Complete answer:
Firstly, we will learn about the concept of hybridization, which can be defined as the mixing of orbitals of the same energy to give new degenerate orbitals.
Boron trifluoride is an inorganic compound. It is a pungent colourless toxic gas which forms white fumes in the moist air. It is a Lewis acid and also a versatile building block for the other boron compounds.
In $BF_{3}$, the central atom boron contains three valence electrons and each electron is shared by fluorine atoms (as shown in the below figure) which results in three sigma bonds and no lone pairs.
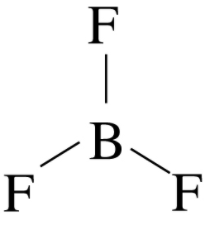
Based on the number of sigma bonds and lone pairs, the steric number will be three. Hence, $BF_{3}$ molecule has $sp^{3}$ hybridization and the shape will be trigonal planar.
Therefore, ‘‘the shape of $BF_{3}$ molecule is trigonal planar’’ is a true statement.
Therefore, the given statement is (A) True.
Note:
We need to remember that the shape does not count lone pairs but the shape is an outcome of a lone pair. For example, in the $NH_{3}$ molecule the structure will be tetrahedral but due to the presence of lone pair the shape of the ammonia molecule comes out to be trigonal pyramidal.
Complete answer:
Firstly, we will learn about the concept of hybridization, which can be defined as the mixing of orbitals of the same energy to give new degenerate orbitals.
Boron trifluoride is an inorganic compound. It is a pungent colourless toxic gas which forms white fumes in the moist air. It is a Lewis acid and also a versatile building block for the other boron compounds.
In $BF_{3}$, the central atom boron contains three valence electrons and each electron is shared by fluorine atoms (as shown in the below figure) which results in three sigma bonds and no lone pairs.

Based on the number of sigma bonds and lone pairs, the steric number will be three. Hence, $BF_{3}$ molecule has $sp^{3}$ hybridization and the shape will be trigonal planar.
Therefore, ‘‘the shape of $BF_{3}$ molecule is trigonal planar’’ is a true statement.
Therefore, the given statement is (A) True.
Note:
We need to remember that the shape does not count lone pairs but the shape is an outcome of a lone pair. For example, in the $NH_{3}$ molecule the structure will be tetrahedral but due to the presence of lone pair the shape of the ammonia molecule comes out to be trigonal pyramidal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




