
The shape of $CI{{F}_{3}}$ molecule is:
A.Triangular planer
B.Pyramidal
C.T-shape
D.Trigonal bipyramidal
Answer
570k+ views
Hint: Using the VSEPR theory, the electron bond pairs and lone pairs on the centre atom help predict the shape of a molecule which is determined by the location of the nuclei and its electrons.
Complete answer:
- Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule.
- To determine the shapes of molecules, we must become familiar with the Lewis electron dot structure. It helps us identify the bond pairs and the lone pairs.
- Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion (VSEPR) theory to determine the molecular geometry and the electron-group geometry.
- According to the VSEPR bond theory, Chlorine trifluoride has 10 electrons around the central chlorine atom.
- This means that there are 5 electron pairs arranged in a trigonal bipyramidal shape with a ${{175}^{0}}$ bond angle.
- But, due to the presence of two equatorial lone pairs, the final structure is T−shaped.
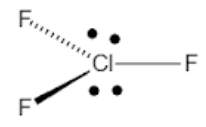
Trigonal bipyramidal
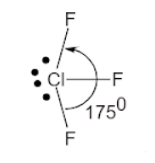
T-shape
Therefore, the answer to the question is (C) T-shape.
Note:
When Cl needs to combine with Fluorine atoms to form ClF3 it needs three unpaired electrons to bond with three F-atoms. Here, one of the paired electrons of Cl in the 3p subshell remains as a lone pair or unpaired. During hybridization, one 3s, three 3p and one of the 3d orbitals participate in the process which leads to the formation of five $s{{p}^{3}}d$ hybrid orbitals.
Complete answer:
- Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule.
- To determine the shapes of molecules, we must become familiar with the Lewis electron dot structure. It helps us identify the bond pairs and the lone pairs.
- Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion (VSEPR) theory to determine the molecular geometry and the electron-group geometry.
- According to the VSEPR bond theory, Chlorine trifluoride has 10 electrons around the central chlorine atom.
- This means that there are 5 electron pairs arranged in a trigonal bipyramidal shape with a ${{175}^{0}}$ bond angle.
- But, due to the presence of two equatorial lone pairs, the final structure is T−shaped.

Trigonal bipyramidal

T-shape
Therefore, the answer to the question is (C) T-shape.
Note:
When Cl needs to combine with Fluorine atoms to form ClF3 it needs three unpaired electrons to bond with three F-atoms. Here, one of the paired electrons of Cl in the 3p subshell remains as a lone pair or unpaired. During hybridization, one 3s, three 3p and one of the 3d orbitals participate in the process which leads to the formation of five $s{{p}^{3}}d$ hybrid orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




