
The shape of $ClO_{3}^{-}$ according to VSEPR model is:
(A) Planar triangle
(B) Pyramidal
(C) Tetrahedral
(D) Square planar
Answer
570k+ views
Hint: The full form of VSEPR theory is valence shell electron pair repulsion theory. To answer this question it is very important to know and understand the postulates of VSEPR theory. With reference to this theory we have to figure out its structure in the first place.
Complete step by step answer:
Valence shell electron pair repulsion (VSEPR) theory is a model used to predict the geometry of the individual molecules from the number of electron pairs surrounding their central atoms.
Postulates of VSEPR theory are:
-The spatial arrangement of the molecules depends upon the number of valence shell electron pairs around the central atom.
-The electron pair surrounding the central atom repels one another and moves apart from each other so that there is no further repulsion between them. As a result, the molecules have minimum energy and maximum stability.
-There are two types of electron pairs, one bond pairs and other lone pairs. The bond pairs of electrons are those shared between two atoms while the lone pair are the valence electron pairs that are not involved in bonding.
-Order of the repulsion between the electron pairs are: $lp-lp$ $>$ $lp-bp$ $>$ $bp-bp$ where, lp is lone pair and bp is bond pair.
The number of lone pairs and the number of bond pairs determines the shape of the given molecule.
Now, let's draw the model of $ClO_{3}^{-}$:
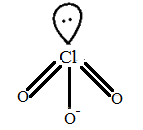
It has 3 $\sigma $- bond and 1 lone pair. It is $A{{B}_{3}}$ type.
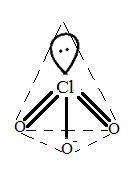
Thus, the shape is pyramidal.
Option B is the correct answer.
Note: The first postulate was derived with reference to Lewis dot structure theory. Even though it failed to explain many things but it was a stepping stone for VSEPR theory. The number of electron pairs is the number of lone pairs and the number of bond pairs determines the shape of the given molecule.
Complete step by step answer:
Valence shell electron pair repulsion (VSEPR) theory is a model used to predict the geometry of the individual molecules from the number of electron pairs surrounding their central atoms.
Postulates of VSEPR theory are:
-The spatial arrangement of the molecules depends upon the number of valence shell electron pairs around the central atom.
-The electron pair surrounding the central atom repels one another and moves apart from each other so that there is no further repulsion between them. As a result, the molecules have minimum energy and maximum stability.
-There are two types of electron pairs, one bond pairs and other lone pairs. The bond pairs of electrons are those shared between two atoms while the lone pair are the valence electron pairs that are not involved in bonding.
-Order of the repulsion between the electron pairs are: $lp-lp$ $>$ $lp-bp$ $>$ $bp-bp$ where, lp is lone pair and bp is bond pair.
The number of lone pairs and the number of bond pairs determines the shape of the given molecule.
Now, let's draw the model of $ClO_{3}^{-}$:

It has 3 $\sigma $- bond and 1 lone pair. It is $A{{B}_{3}}$ type.

Thus, the shape is pyramidal.
Option B is the correct answer.
Note: The first postulate was derived with reference to Lewis dot structure theory. Even though it failed to explain many things but it was a stepping stone for VSEPR theory. The number of electron pairs is the number of lone pairs and the number of bond pairs determines the shape of the given molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




