
The shape of $I{F_7}$ molecule is:
(A) Pentagonal bipyramidal
(B) Trigonal bipyramidal
(C) Octagonal
(D) T-shape
Answer
584.4k+ views
Hint: To answer this question, we must first understand the VSEPR theory. The Valence Shell Electron Pair Repulsion Theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom.
Complete step by step solution:
-By VSEPR Theory we can predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. It is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized. This arrangement of the atom helps us determine the geometry of the resulting molecule.
-Iodine heptafluoride as we can see contains a single iodine atom attached to seven fluorine atoms which give it a molecular formula of $I{F_7}$. The molecule $I{F_7}$ does not occur in nature and is only possible through orbital hybridization. It is an intermediary molecule in some chemical reactions and is a byproduct of dioxygenyl hexafluoroplatinate to prepare other platinum-containing compounds. It can also be produced when using potassium fluoride in iodine pentafluoride.
-From VSEPR theory, we see that $I{F_7}$ has an $s{p^3}{d^3}$ hybridization and a coordination number of 7. Hence it has pentagonal bipyramidal geometry.
It can be better understood from the following diagram:
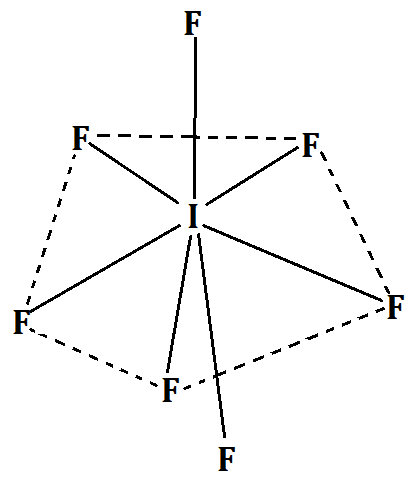
Hence, the correct answer is option (A) Pentagonal bipyramidal.
Note: We should note that VSEPR theory can be used only to predict the geometry of the molecules of many compounds accurately but not the shape. For example, in spite of having pentagonal bipyramidal geometry, Iodine heptafluoride has a distorted octahedral shape. But this theory is extremely essential since by predicting the geometry, we can ultimately predict the reactions the molecule would go through.
Complete step by step solution:
-By VSEPR Theory we can predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. It is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized. This arrangement of the atom helps us determine the geometry of the resulting molecule.
-Iodine heptafluoride as we can see contains a single iodine atom attached to seven fluorine atoms which give it a molecular formula of $I{F_7}$. The molecule $I{F_7}$ does not occur in nature and is only possible through orbital hybridization. It is an intermediary molecule in some chemical reactions and is a byproduct of dioxygenyl hexafluoroplatinate to prepare other platinum-containing compounds. It can also be produced when using potassium fluoride in iodine pentafluoride.
-From VSEPR theory, we see that $I{F_7}$ has an $s{p^3}{d^3}$ hybridization and a coordination number of 7. Hence it has pentagonal bipyramidal geometry.
It can be better understood from the following diagram:

Hence, the correct answer is option (A) Pentagonal bipyramidal.
Note: We should note that VSEPR theory can be used only to predict the geometry of the molecules of many compounds accurately but not the shape. For example, in spite of having pentagonal bipyramidal geometry, Iodine heptafluoride has a distorted octahedral shape. But this theory is extremely essential since by predicting the geometry, we can ultimately predict the reactions the molecule would go through.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




