
The shape of methane molecule is
A ) pentahedral
B ) hexahedral
C ) octahedral
D ) tetrahedral
Answer
583.8k+ views
Hint: Tetrahedral, pentahedral, and octahedral geometry is associated with 4, 5, and 6 bonding domains respectively. Here bonding domains include bond pairs of electrons and lone pairs of electrons. Thus, if four bond pairs and zero lone pairs of electrons are present, tetrahedral geometry is obtained. Similarly, if 6 bond pairs and zero lone pairs of electrons are obtained. Octahedral geometry is obtained.
Complete step by step answer:
> Methane is the saturated hydrocarbon. It is the first member of alkane homologous series. It only contains single bonds. It does not contain double or triple bonds. The chemical formula of methane is \[{\text{C}}{{\text{H}}_4}\]. One molecule of methane contains one carbon atom and four hydrogen atoms. The carbon atom is the central atom. It forms four single bonds with four hydrogen atoms.
> The atomic number of carbon is 6. It has 2 electrons in the K shell and 4 electrons in the L shell. The electronic configuration of carbon is \[1{s^2}2{s^2}2{p^2}\]. There are four electrons in the valence shell of a carbon atom. The atomic number of hydrogen is 1. It has one electron in the K shell. The electronic configuration of hydrogen is \[1{s^1}\]. The valence shell of hydrogen contains one electron.
> Carbon atom undergoes \[s{p^3}\] hybridisation in which \[2s,2{p_x},2{p_y},2{p_z}\] orbitals of carbon undergo hybridisation to form four equivalent degenerate \[s{p^3}\] hybrid orbitals. The number of hybrid orbitals is equal to the number of atomic orbitals that participate in hybridisation. The number of hybrid orbitals is also equal to the number of bonds around the carbon atom in methane, as the number of lone pairs of electrons around the carbon atom is zero. \[s{p^3}\] hybridisation is associated with tetrahedral geometry in which carbon atom is present at the centre of the tetrahedron and four hydrogen atoms are present at four corners of regular tetrahedron.
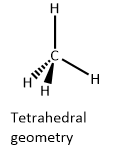
Hence, the correct option is D ) tetrahedral.
Note: If the central atom has no lone pairs of electrons, then tetrahedral geometry is obtained when the central atom has four bonds. Pentahedral geometry is obtained when a central atom has five bonds. Octahedral geometry is obtained when a central atom has six bonds. Thus, for the molecules, in which the central atom has no lone pairs of electrons, it is possible to determine the geometry by counting the number of bonds around the central atom.
Complete step by step answer:
> Methane is the saturated hydrocarbon. It is the first member of alkane homologous series. It only contains single bonds. It does not contain double or triple bonds. The chemical formula of methane is \[{\text{C}}{{\text{H}}_4}\]. One molecule of methane contains one carbon atom and four hydrogen atoms. The carbon atom is the central atom. It forms four single bonds with four hydrogen atoms.
> The atomic number of carbon is 6. It has 2 electrons in the K shell and 4 electrons in the L shell. The electronic configuration of carbon is \[1{s^2}2{s^2}2{p^2}\]. There are four electrons in the valence shell of a carbon atom. The atomic number of hydrogen is 1. It has one electron in the K shell. The electronic configuration of hydrogen is \[1{s^1}\]. The valence shell of hydrogen contains one electron.
> Carbon atom undergoes \[s{p^3}\] hybridisation in which \[2s,2{p_x},2{p_y},2{p_z}\] orbitals of carbon undergo hybridisation to form four equivalent degenerate \[s{p^3}\] hybrid orbitals. The number of hybrid orbitals is equal to the number of atomic orbitals that participate in hybridisation. The number of hybrid orbitals is also equal to the number of bonds around the carbon atom in methane, as the number of lone pairs of electrons around the carbon atom is zero. \[s{p^3}\] hybridisation is associated with tetrahedral geometry in which carbon atom is present at the centre of the tetrahedron and four hydrogen atoms are present at four corners of regular tetrahedron.

Hence, the correct option is D ) tetrahedral.
Note: If the central atom has no lone pairs of electrons, then tetrahedral geometry is obtained when the central atom has four bonds. Pentahedral geometry is obtained when a central atom has five bonds. Octahedral geometry is obtained when a central atom has six bonds. Thus, for the molecules, in which the central atom has no lone pairs of electrons, it is possible to determine the geometry by counting the number of bonds around the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




