
The shape of $Xe{{F}_{6}}$ is
(A) Square planar
(B) Distorted octahedral
(C) Square pyramidal
(D) Pyramidal
Answer
522.6k+ views
Hint: Find out the location of xenon in the periodic table and then write its ground state electronic configuration. The given molecule in question whose geometry we need to find is xenon hexafluoride. So 6 fluorine atoms have to form bonds with xenon. Write the valence shell electrons of both xenon and fluorine and then find out the number of electron pairs present in this molecule and then draw the Lewis structure. Then from the structure write hybridization and find out the geometry. Keep in mind VSEPR theory while assigning the geometry.
Complete step by step answer:
- Xenon is a noble gas element which belongs to the fifth period.
- Let’s write the electronic configuration of Xenon.
\[Xe=[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}\]
- There are 7 valence shell electrons in each fluorine and eight valence shell electrons in xenon atom. So, the number of electrons present is $8+\left( 6\times 7 \right)=50$ and the total number of electron pairs present is 25.
- The Lewis structure of Xenon hexafluoride is,
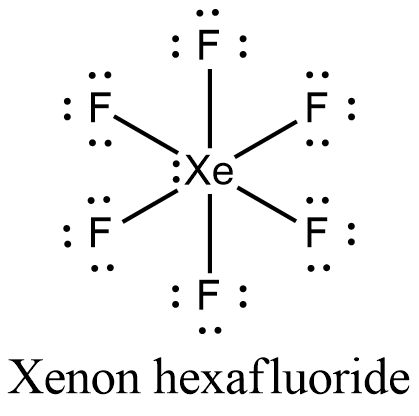
- In xenon, there are eight electrons in its valence shell. It also has an empty 5d orbital. There are six fluorine atoms in xenon hexafluoride, six covalent bonds are formed. Xenon undergoes $s{{p}^{3}}{{d}^{3}}$ hybridization to form seven $s{{p}^{3}}{{d}^{3}}$ hybrid orbitals. Six $s{{p}^{3}}{{d}^{3}}$ orbitals have one electron each and one $s{{p}^{3}}{{d}^{3}}$ contain a lone pair of electrons. The six unpaired electrons get paired with six electrons from six fluorine atoms to form xenon hexafluoride, $Xe{{F}_{6}}$.
- Due to the presence of one lone pair of electrons, the will be lone-pair –bond-pair repulsion in the molecule and the geometry will be distorted octahedral.
So, the correct answer is “Option B”.
Note: Remember xenon hexafluoride has a distorted octahedral geometry. Due to the presence of one lone pair on a xenon atom, the octahedral geometry is distorted. Lone-pair-lone-pair repulsion is more than lone-pair-bond-pair repulsion which is in turn greater than bond-pair-bond-pair repulsion.
Complete step by step answer:
- Xenon is a noble gas element which belongs to the fifth period.
- Let’s write the electronic configuration of Xenon.
\[Xe=[Kr]4{{d}^{10}}5{{s}^{2}}5{{p}^{6}}\]
- There are 7 valence shell electrons in each fluorine and eight valence shell electrons in xenon atom. So, the number of electrons present is $8+\left( 6\times 7 \right)=50$ and the total number of electron pairs present is 25.
- The Lewis structure of Xenon hexafluoride is,

- In xenon, there are eight electrons in its valence shell. It also has an empty 5d orbital. There are six fluorine atoms in xenon hexafluoride, six covalent bonds are formed. Xenon undergoes $s{{p}^{3}}{{d}^{3}}$ hybridization to form seven $s{{p}^{3}}{{d}^{3}}$ hybrid orbitals. Six $s{{p}^{3}}{{d}^{3}}$ orbitals have one electron each and one $s{{p}^{3}}{{d}^{3}}$ contain a lone pair of electrons. The six unpaired electrons get paired with six electrons from six fluorine atoms to form xenon hexafluoride, $Xe{{F}_{6}}$.
- Due to the presence of one lone pair of electrons, the will be lone-pair –bond-pair repulsion in the molecule and the geometry will be distorted octahedral.
So, the correct answer is “Option B”.
Note: Remember xenon hexafluoride has a distorted octahedral geometry. Due to the presence of one lone pair on a xenon atom, the octahedral geometry is distorted. Lone-pair-lone-pair repulsion is more than lone-pair-bond-pair repulsion which is in turn greater than bond-pair-bond-pair repulsion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




