
The shapes of \[I{{F}_{5}}\] and \[I{{F}_{7}}\] are respectively?
a.) square pyramidal and pentagonal bipyramidal
b.) octahedral and pyramidal
c.) trigonal bipyramidal and square antiprismatic
d.) distorted square planar and distorted octahedral
Answer
601.5k+ views
Hint: The shape of a molecule affects how it interacts with other molecules and that in turn can give rise to many interesting phenomena. It can be best described by VSEPR theory.
Complete step by step solution:
VSEPR stands for Valence Shell Electron Pair Repulsion theory. This theory assumes that the atoms within the molecules are arranged in such a manner as to minimize repulsion between electrons in the valence shell of that atom.
If there is a polyatomic molecule, one of the constituent atoms is identified as the central atom and all the other atoms are linked to it.
For predicting the shape of the molecule, the least electronegative atom must be considered as the central atom and the valence electron of the central atom must be counted.
The total number of electrons of the other atoms which are used in the bond with the central atom should also be counted.
For example: Oxygen will form a double bond with the central atom as its valency is 2. So, the number of electrons involved in the bond formation is 4.
Iodine has 7 valence electrons and it is the least electronegative atom. So, we will take Iodine as the central atom.
In case of \[I{{F}_{5}}\] out of the 7 valence electrons of Iodine, 5 will be occupied by the 5 Fluorine atoms, forming a single bond each. Thus, Iodine has a lone pair of electrons remaining.
Now the bonds will try to arrange themselves in a manner to minimize the repulsion between the electrons.
5 bonds and 1 lone pair represents octahedral geometry and the molecular shape will be square pyramidal. The angle between the axial bonds will be 180 degrees and the square planar adjacent fluorine atoms will have an angle of 90 degrees.
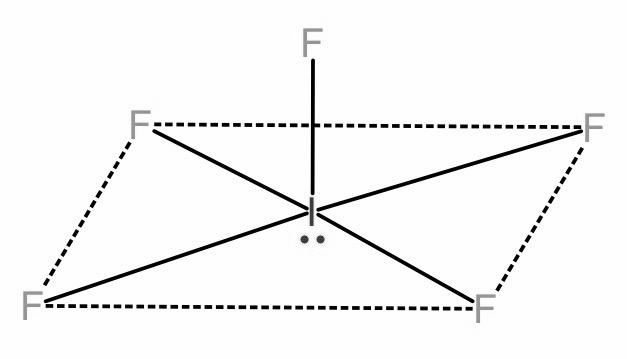
\[I{{F}_{5}}\]
Similarly, in \[I{{F}_{7}}\] all the 7 valence electrons of the Iodine will be occupied by the 7 of the fluorine atoms. Thus, iodine will not have any lone pair electrons.
7 bonds and 0 lone pairs represents a pentagonal bipyramidal shape. The angle between the axial bonds will be 180 degrees and planar fluorine bonds will have an angle 72 degrees.
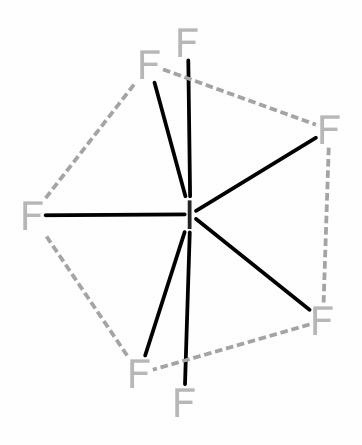
\[I{{F}_{7}}\]
Therefore, from the above statements the correct answer is (a).
Note: The angle between the adjacent bonds can be altered because of the repulsion between the bond pair and the Lone pairs, so the shape of the \[I{{F}_{5}}\] can sometimes be distorted square pyramidal Because of the presence of the lone pair electrons.
Complete step by step solution:
VSEPR stands for Valence Shell Electron Pair Repulsion theory. This theory assumes that the atoms within the molecules are arranged in such a manner as to minimize repulsion between electrons in the valence shell of that atom.
If there is a polyatomic molecule, one of the constituent atoms is identified as the central atom and all the other atoms are linked to it.
For predicting the shape of the molecule, the least electronegative atom must be considered as the central atom and the valence electron of the central atom must be counted.
The total number of electrons of the other atoms which are used in the bond with the central atom should also be counted.
For example: Oxygen will form a double bond with the central atom as its valency is 2. So, the number of electrons involved in the bond formation is 4.
Iodine has 7 valence electrons and it is the least electronegative atom. So, we will take Iodine as the central atom.
In case of \[I{{F}_{5}}\] out of the 7 valence electrons of Iodine, 5 will be occupied by the 5 Fluorine atoms, forming a single bond each. Thus, Iodine has a lone pair of electrons remaining.
Now the bonds will try to arrange themselves in a manner to minimize the repulsion between the electrons.
5 bonds and 1 lone pair represents octahedral geometry and the molecular shape will be square pyramidal. The angle between the axial bonds will be 180 degrees and the square planar adjacent fluorine atoms will have an angle of 90 degrees.

\[I{{F}_{5}}\]
Similarly, in \[I{{F}_{7}}\] all the 7 valence electrons of the Iodine will be occupied by the 7 of the fluorine atoms. Thus, iodine will not have any lone pair electrons.
7 bonds and 0 lone pairs represents a pentagonal bipyramidal shape. The angle between the axial bonds will be 180 degrees and planar fluorine bonds will have an angle 72 degrees.

\[I{{F}_{7}}\]
Therefore, from the above statements the correct answer is (a).
Note: The angle between the adjacent bonds can be altered because of the repulsion between the bond pair and the Lone pairs, so the shape of the \[I{{F}_{5}}\] can sometimes be distorted square pyramidal Because of the presence of the lone pair electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




