
The \[SiC{l_4}\] molecule is nonpolar and chlorine is more electronegative than silicon. From this information alone it can be deduced that:
1. \[Si-Cl\] bond is nonpolar
2. \[SiC{l_4}\] molecule is planar
3. \[SiC{l_4}\] molecule is symmetrical
A. If 1,2 and 3 are correct
B. If only 1 and 2 are correct
C. If only 2 and 3 are correct
D. If only 1 is correct
E. If only 3 is correct
Answer
580.8k+ views
Hint: To solve this question, we must first understand the principles behind the formation of polar and non – polar bonds and molecules. Then on the basis of that discussion, we can deduce the correct answer from the options.
Complete step by step answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Polar bonds are formed when there is a significant difference in the electronegativities of the atoms that are forming the covalent bonds. Because of this, the atom with higher electronegativity tends to pull the electron cloud of the bond towards itself. Hence, there is a shift in the charge between the atoms and this results in the formation of a dipole.
On the other hand, non – polar bonds are of two types:
When the electronegativity difference between the bonding atoms is not significant enough, there is no shift of the electron cloud and the charge is equally distributed over both the atoms.
Another way in which non – polar bonds are formed is when two polar bonds having equal dipole moments are placed right across each other in the compound. This cancels out the dipole moment created by either of the two bonds and hence the compound in general becomes a non – polar compound.
Now, in the question we have been given that there is an electronegativity difference between Si and Cl. Hence, their bonds are polar bonds. The only way their compound is non – polar is when all the \[Si-Cl\] bonds are placed exactly opposite each other. This means that the molecule needs to have symmetric geometry.
Hence, Option E is the correct option.
Note:
The actual geometric structure of \[SiC{l_4}\] is tetrahedral. The Si atom is placed at the centre and all the Cl atoms are placed around it. Their dipoles cancel each other out to make \[SiC{l_4}\] a non – polar compound. The molecular structure of \[SiC{l_4}\] can be given as:
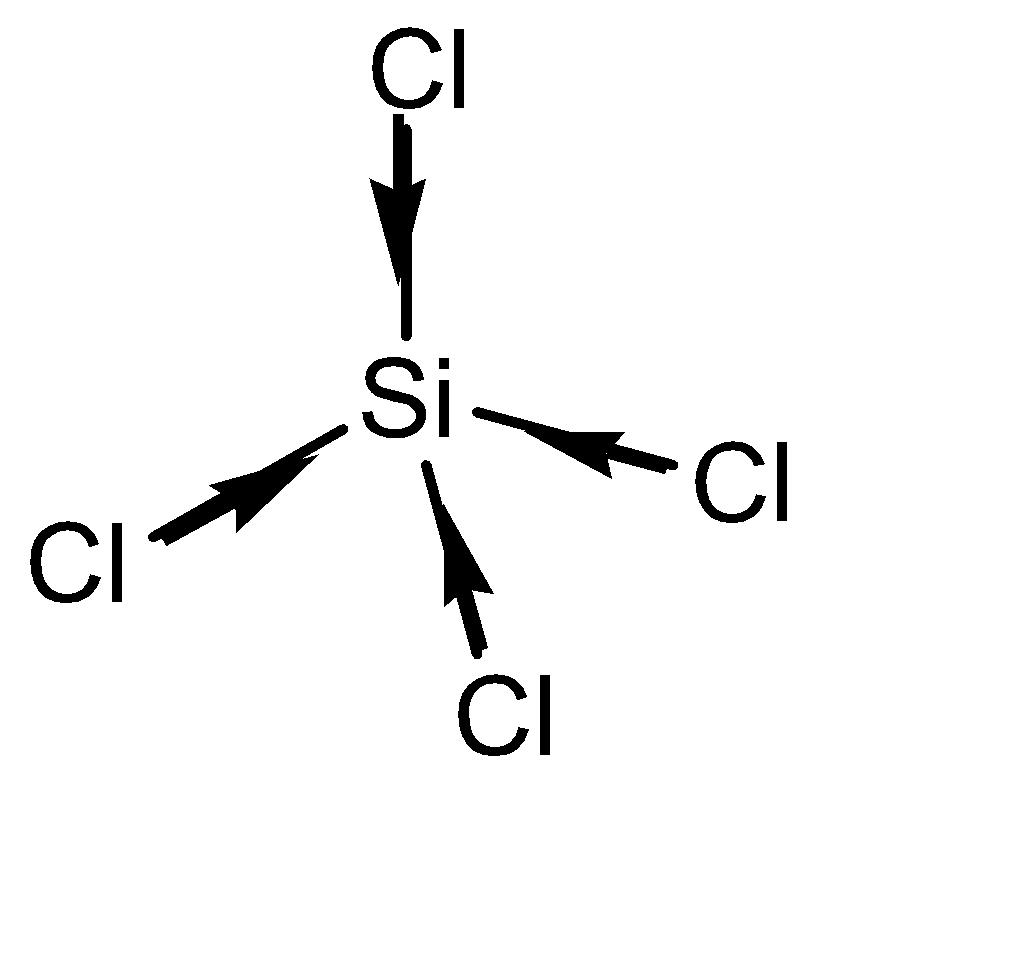
Complete step by step answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
Polar bonds are formed when there is a significant difference in the electronegativities of the atoms that are forming the covalent bonds. Because of this, the atom with higher electronegativity tends to pull the electron cloud of the bond towards itself. Hence, there is a shift in the charge between the atoms and this results in the formation of a dipole.
On the other hand, non – polar bonds are of two types:
When the electronegativity difference between the bonding atoms is not significant enough, there is no shift of the electron cloud and the charge is equally distributed over both the atoms.
Another way in which non – polar bonds are formed is when two polar bonds having equal dipole moments are placed right across each other in the compound. This cancels out the dipole moment created by either of the two bonds and hence the compound in general becomes a non – polar compound.
Now, in the question we have been given that there is an electronegativity difference between Si and Cl. Hence, their bonds are polar bonds. The only way their compound is non – polar is when all the \[Si-Cl\] bonds are placed exactly opposite each other. This means that the molecule needs to have symmetric geometry.
Hence, Option E is the correct option.
Note:
The actual geometric structure of \[SiC{l_4}\] is tetrahedral. The Si atom is placed at the centre and all the Cl atoms are placed around it. Their dipoles cancel each other out to make \[SiC{l_4}\] a non – polar compound. The molecular structure of \[SiC{l_4}\] can be given as:

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




