
The sigma bond energy of \[{\text{C}} - {\text{H}}\] bond in \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{}}\]is:
A.99 kcal
B.140 kcal
C.200 kcal
D.60 kcal
Answer
577.8k+ views
Hint: The first bond formed between any two atoms is the sigma bond. The sigma bond energy is merely the energy required to form or break that bond.
Complete step by step answer:
The sigma bond is the strongest covalent bond formed by the head on overlap between the atomic orbitals. They are simply the first bond formed between any two atoms. The ${\text{C}} - {\text{H}}$ bond in ethane ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{}}$ is known as the sigma bond. It is denoted as σ. The structure of ethane s shown below:
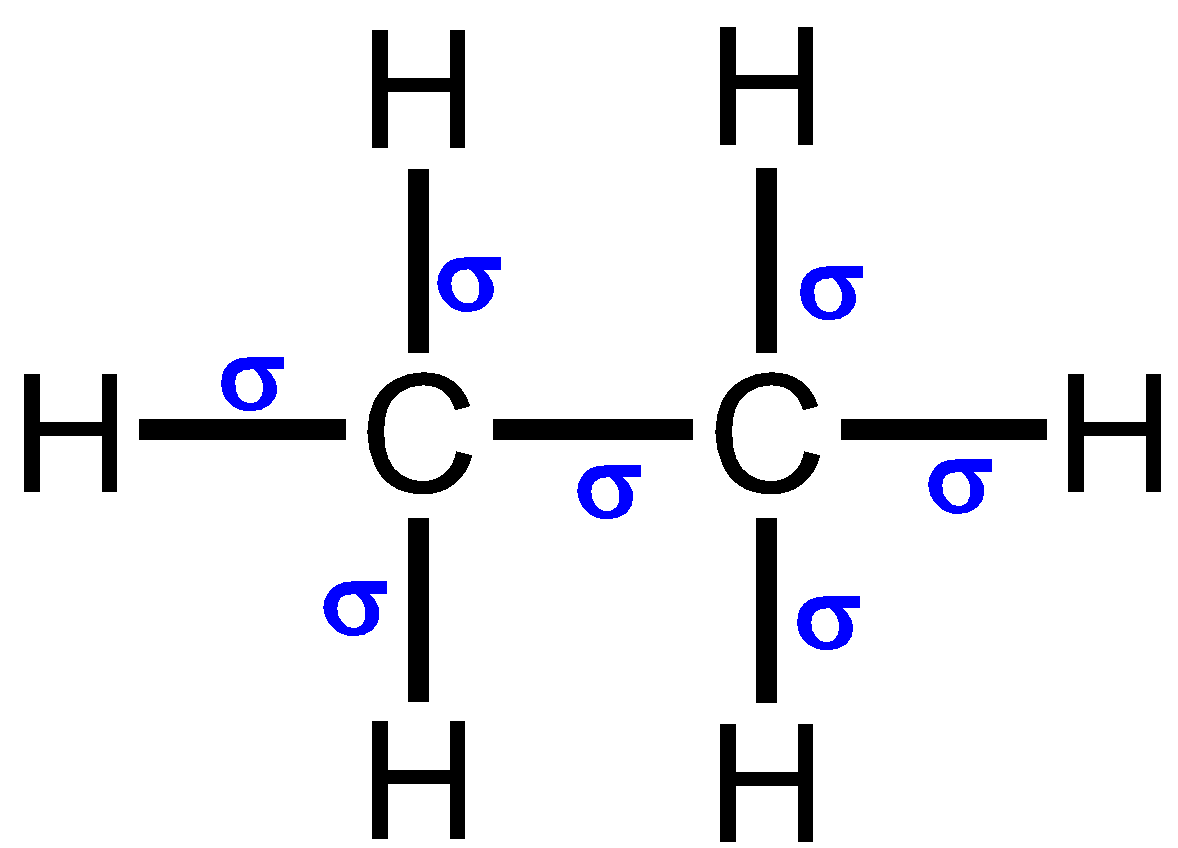
The energy required to form the sigma bond or break that bond is known as sigma bond energy. They are generally called bond dissociation energy. It can be expressed in kcal or kJ/mol. The bond energy is usually calculated at the room temperature. The stability of a bond is directly related to its bond energy. As the bond energy increases stability increases. This in turn decreases the reactivity of the bond. They are known to depict the strength of the chemical bond.
The sigma bond energy in \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{}}\] is 99 kcal. They are calculated from the spectroscopic determination of the energy levels. The ethylene has a bond dissociation energy of 174 kcal/mol.
So, the correct option is A.
Note:
The sigma bond energy can also be expressed in kJ/mol. The conversion factor is given below:
1 ${\text{ kcal/mol = 4}}{\text{.184 kJ/mol}}$
Thus, the sigma bond energy in \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{}}\]= 99 kcal = 413 kJ/mol.
Complete step by step answer:
The sigma bond is the strongest covalent bond formed by the head on overlap between the atomic orbitals. They are simply the first bond formed between any two atoms. The ${\text{C}} - {\text{H}}$ bond in ethane ${{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{}}$ is known as the sigma bond. It is denoted as σ. The structure of ethane s shown below:

The energy required to form the sigma bond or break that bond is known as sigma bond energy. They are generally called bond dissociation energy. It can be expressed in kcal or kJ/mol. The bond energy is usually calculated at the room temperature. The stability of a bond is directly related to its bond energy. As the bond energy increases stability increases. This in turn decreases the reactivity of the bond. They are known to depict the strength of the chemical bond.
The sigma bond energy in \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{}}\] is 99 kcal. They are calculated from the spectroscopic determination of the energy levels. The ethylene has a bond dissociation energy of 174 kcal/mol.
So, the correct option is A.
Note:
The sigma bond energy can also be expressed in kJ/mol. The conversion factor is given below:
1 ${\text{ kcal/mol = 4}}{\text{.184 kJ/mol}}$
Thus, the sigma bond energy in \[{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{}}\]= 99 kcal = 413 kJ/mol.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




