
The species that does not contain peroxide ion is:
(a)- $Pb{{O}_{2}}$
(b)- ${{H}_{2}}{{O}_{2}}$
(c)- $Sr{{O}_{2}}$
(d)- $Ba{{O}_{2}}$
Answer
576.9k+ views
Hint: If the compound or molecules have two oxygen atoms attached by a single bond then the compound is said to have peroxide ion. The structure is $-O-O-$.
Complete step by step answer:
If the compound or molecules have two oxygen atoms attached by a single bond then the compound is said to have peroxide ion. The structure is $-O-O-$. There is -1 oxidation state of oxygen in peroxide. If there is a formation of hydrogen peroxide when the metal oxide reacts with dilute acids then they are called peroxides.
-So in $Pb{{O}_{2}}$, there are two oxygen atoms and one lead atom, both the oxygen atoms are attached to the lead atom with double bonds. The structure is given below:
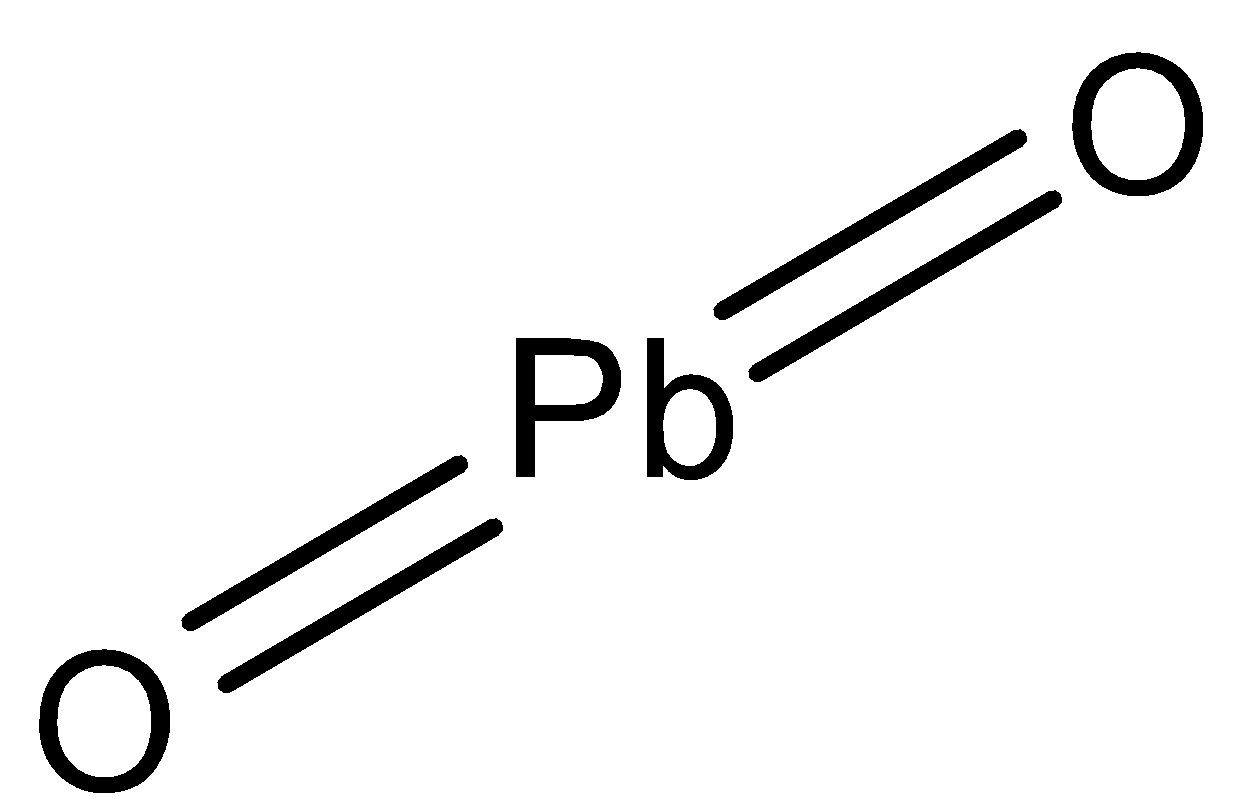
So, it is not peroxide and the oxidation state of oxygen in $Pb{{O}_{2}}$ is -2, but the oxidation state of oxygen in peroxide is -1.
-So in ${{H}_{2}}{{O}_{2}}$, there are two oxygen and two hydrogen atoms, each hydrogen atom is attached to one oxygen atom and both the oxygen atoms are joined together by a single bond. The structure is given below:
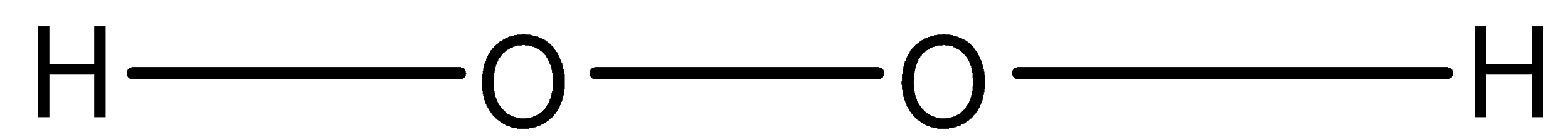
So, it is peroxide and the oxidation state of oxygen in ${{H}_{2}}{{O}_{2}}$ is -1 and that of hydrogen is +1.
-In $Sr{{O}_{2}}$, there are two oxygen atoms and one strontium atom and they are in ionic form i.e., oxygen atoms are in peroxide form and strontium is in cation form. The structure is given below:
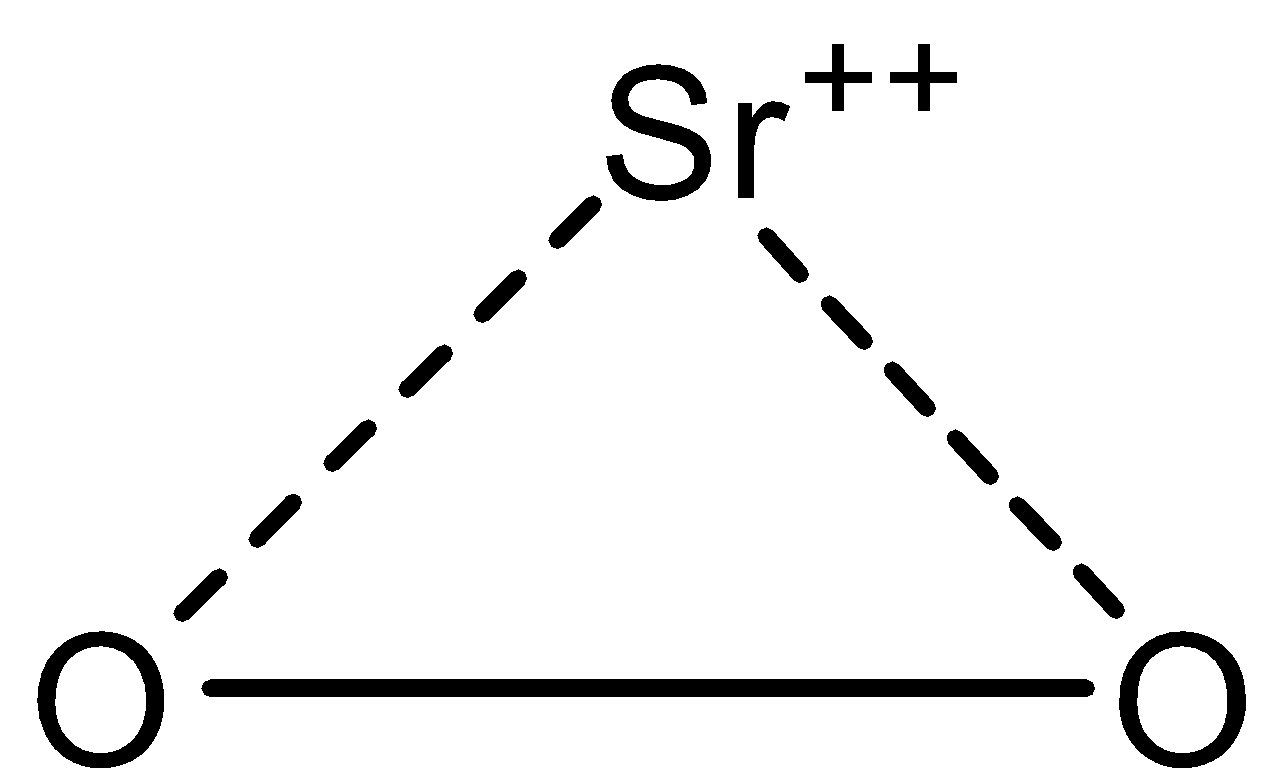
So it is peroxide and the oxidation state of oxygen in $Sr{{O}_{2}}$ is -1 and that of strontium metal is +2.
-In $Ba{{O}_{2}}$, there are two oxygen atoms and one barium atom and they are in ionic form i.e., oxygen atoms are in peroxide form and barium is in cation form. The structure is given below:
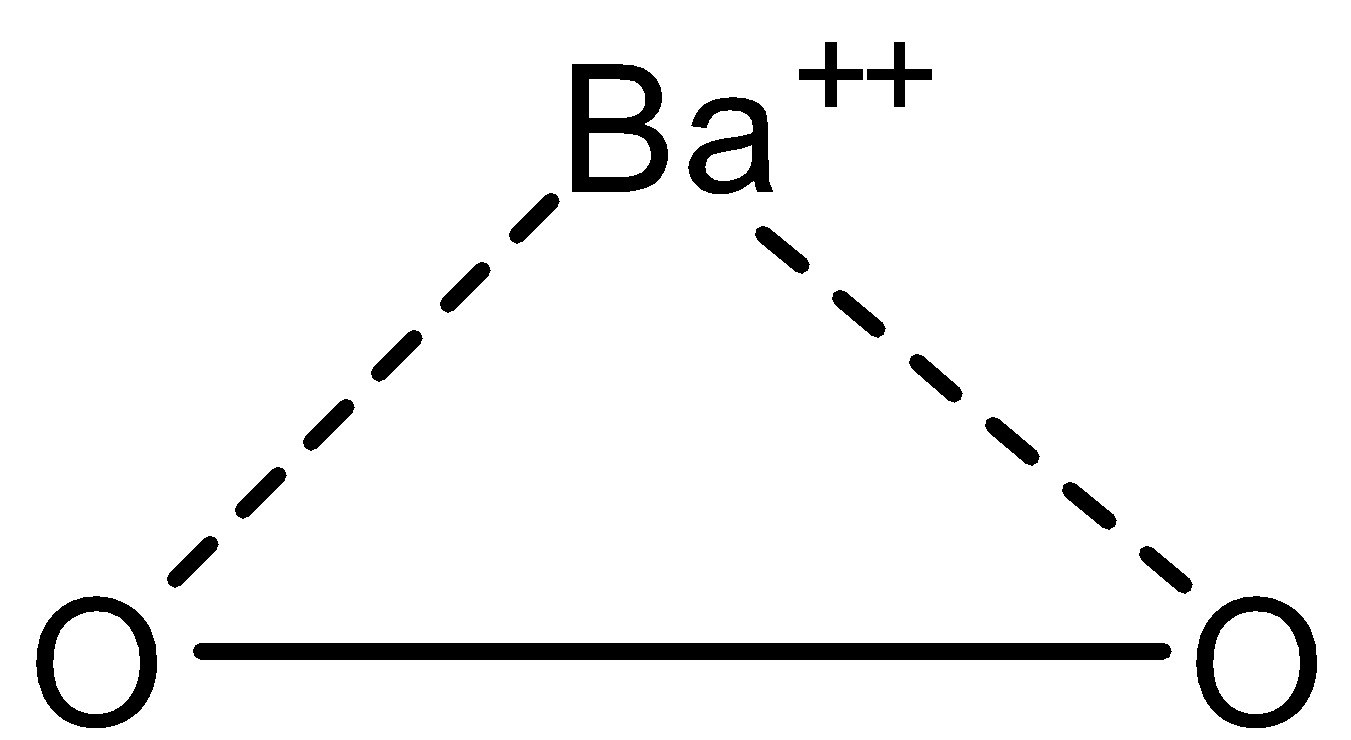
So it is peroxide and the oxidation state of oxygen in $Ba{{O}_{2}}$ is -1 and that of barium metal is +2.
Therefore, the correct answer is option (a)- $Pb{{O}_{2}}$.
Note: In peroxide the bond between the oxygen atoms is covalent. In the ionic form, we can write the peroxide ion as $O_{2}^{2-}$. The main use of peroxide is bleaching.
Complete step by step answer:
If the compound or molecules have two oxygen atoms attached by a single bond then the compound is said to have peroxide ion. The structure is $-O-O-$. There is -1 oxidation state of oxygen in peroxide. If there is a formation of hydrogen peroxide when the metal oxide reacts with dilute acids then they are called peroxides.
-So in $Pb{{O}_{2}}$, there are two oxygen atoms and one lead atom, both the oxygen atoms are attached to the lead atom with double bonds. The structure is given below:

So, it is not peroxide and the oxidation state of oxygen in $Pb{{O}_{2}}$ is -2, but the oxidation state of oxygen in peroxide is -1.
-So in ${{H}_{2}}{{O}_{2}}$, there are two oxygen and two hydrogen atoms, each hydrogen atom is attached to one oxygen atom and both the oxygen atoms are joined together by a single bond. The structure is given below:
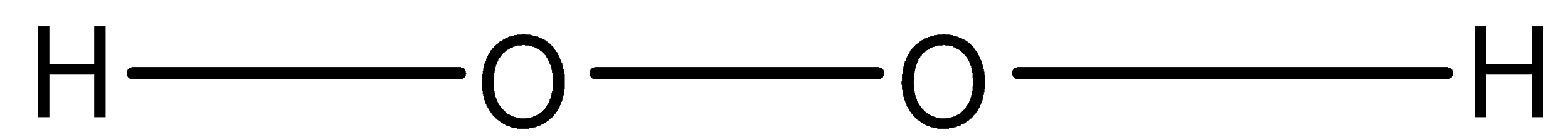
So, it is peroxide and the oxidation state of oxygen in ${{H}_{2}}{{O}_{2}}$ is -1 and that of hydrogen is +1.
-In $Sr{{O}_{2}}$, there are two oxygen atoms and one strontium atom and they are in ionic form i.e., oxygen atoms are in peroxide form and strontium is in cation form. The structure is given below:

So it is peroxide and the oxidation state of oxygen in $Sr{{O}_{2}}$ is -1 and that of strontium metal is +2.
-In $Ba{{O}_{2}}$, there are two oxygen atoms and one barium atom and they are in ionic form i.e., oxygen atoms are in peroxide form and barium is in cation form. The structure is given below:

So it is peroxide and the oxidation state of oxygen in $Ba{{O}_{2}}$ is -1 and that of barium metal is +2.
Therefore, the correct answer is option (a)- $Pb{{O}_{2}}$.
Note: In peroxide the bond between the oxygen atoms is covalent. In the ionic form, we can write the peroxide ion as $O_{2}^{2-}$. The main use of peroxide is bleaching.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




