
The structural formula of ethyl ethanoate is:
1.
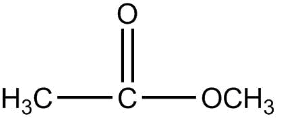
2.
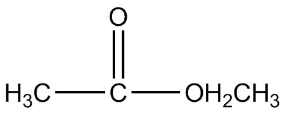
3.
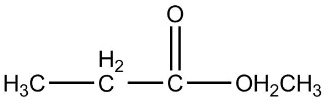
4.
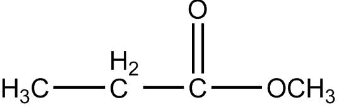




Answer
537.3k+ views
Hint: Ethyl acetic acid derivation, otherwise called ethyl ethanoate is one natural compound and is basically utilized as a dissolvable in various responses. Ethyl acetic acid derivation is acquired from the immediate esterification of ethyl liquor with acidic corrosive, a cycle which includes blending acidic corrosive in with an abundance of ethyl liquor and adding a modest quantity of sulphuric corrosive.
Complete step by step solution:
Now, first of all, understand what is ethyl ethanoate, we will identify the functional group in this compound as we can see that it is clearly an ester as an ester contains two alkyl group on both the sides of a carbon-oxygen bond and the general formula of ether is $RCOOR$. Since the prefix in the compound is ethyl that means the ethyl group is attached to the carbon of the functional group of ester.
So the formula of ethyl is ${C_2}{H_5}$ now the prefix used for the suffix oate is ethane, which means the oxygen of ester functional group is attached with one carbon as one carbon is contained in the general formula already, that means methyl is attached on one side of the functional group.
Therefore the final structure of the ethyl ethanoate compound will be
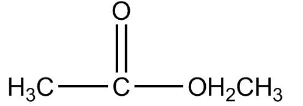
So the correct answer is 2.
Note: Ethyl acetic acid derivation is just feebly Lewis fundamental. It structures adducts with I2, phenol, and bis (hexafluoroacetylacetonate) copper (II). Ethyl acetic acid derivation hydrolyses to give acidic corrosive and ethanol. Bases quicken the hydrolysis, which is dependent upon the Fischer balance referenced previously. In the research center, and ordinarily for illustrative purposes just, ethyl esters are commonly hydrolyzed in a two-venture measure beginning with a stoichiometric measure of a solid base, for example, sodium hydroxide.
Complete step by step solution:
Now, first of all, understand what is ethyl ethanoate, we will identify the functional group in this compound as we can see that it is clearly an ester as an ester contains two alkyl group on both the sides of a carbon-oxygen bond and the general formula of ether is $RCOOR$. Since the prefix in the compound is ethyl that means the ethyl group is attached to the carbon of the functional group of ester.
So the formula of ethyl is ${C_2}{H_5}$ now the prefix used for the suffix oate is ethane, which means the oxygen of ester functional group is attached with one carbon as one carbon is contained in the general formula already, that means methyl is attached on one side of the functional group.
Therefore the final structure of the ethyl ethanoate compound will be

So the correct answer is 2.
Note: Ethyl acetic acid derivation is just feebly Lewis fundamental. It structures adducts with I2, phenol, and bis (hexafluoroacetylacetonate) copper (II). Ethyl acetic acid derivation hydrolyses to give acidic corrosive and ethanol. Bases quicken the hydrolysis, which is dependent upon the Fischer balance referenced previously. In the research center, and ordinarily for illustrative purposes just, ethyl esters are commonly hydrolyzed in a two-venture measure beginning with a stoichiometric measure of a solid base, for example, sodium hydroxide.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




