
The structural unit present in pyro silicates is:
A) \[S{{i}_{3}}{{O}_{9}}^{6-}\]
B) \[Si{{O}_{4}}^{2-}\]
C) $S{{i}_{2}}{{O}_{7}}^{6-}$
D) ${{\left( Si{{O}_{5}}^{2-} \right)}_{n}}$
Answer
571.8k+ views
Hint: The tetrahedral structural unit of silicate has the formulae, $Si{{O}_{4}}^{4-}$. Silicates units are completely made of Si and O atoms only.
Complete step by step answer:
Silicates are those minerals which contain tetrahedral units of formulae $Si{{O}_{4}}^{4-}$, and these tetrahedral units combine in different fashion to yield different types of silicates.
Pyro silicates two tetrahedral units of silicates combined by sharing an Oxygen atom with two units. Or we can say that the tetrahedral silicate unit joins by the removal of an $O$ atom and the units are combined at the corner of the $O$ atom. So the formulae becomes $S{{i}_{2}}{{O}_{7}}^{6-}$.
The structure of pyro silicate is,
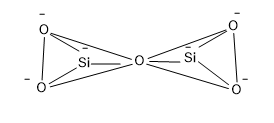
So from the structure it is clear that, a Si atom which is surrounded by four O atoms having a negative charge that is the silicate tetrahedral structure of formulae $Si{{O}_{4}}^{4-}$ and two units of silicate units are combined at the corner of one of the O atom and in this combination one O is lost.
So the correct option for the answer is option (C).
Additional information:
On the basis of tetrahedral units of silicate combines, it is classified into:
Ortho silicates -$\left( Si{{O}_{4}}^{4-} \right)$
Pyro silicates - ($S{{i}_{2}}{{O}_{7}}^{6-}$)
Cyclic silicates-${{\left( Si{{O}_{3}} \right)}_{n}}^{2n-}$
Chain silicates-${{\left( Si{{O}_{3}} \right)}_{n}}^{2n-}$
Double chain silicates -${{\left( S{{i}_{4}}{{O}_{11}} \right)}_{n}}^{6-n}$
Sheet silicates-${{\left( S{{i}_{2}}{{O}_{5}} \right)}_{n}}^{2-n}$, with discrete structural units.
Note: Example of silicates is Thortveitite with the formulae, \[S{{c}_{2}}S{{i}_{2}}{{O}_{7}}\]. If instead of pyro silicate Thortveitite or if its formulae was give and asked to answer the structural unit of it then we may write, as Thortveitite comes under the classification of Pyro silicates the structural unit will be $S{{i}_{2}}{{O}_{7}}^{6-}$
Complete step by step answer:
Silicates are those minerals which contain tetrahedral units of formulae $Si{{O}_{4}}^{4-}$, and these tetrahedral units combine in different fashion to yield different types of silicates.
Pyro silicates two tetrahedral units of silicates combined by sharing an Oxygen atom with two units. Or we can say that the tetrahedral silicate unit joins by the removal of an $O$ atom and the units are combined at the corner of the $O$ atom. So the formulae becomes $S{{i}_{2}}{{O}_{7}}^{6-}$.
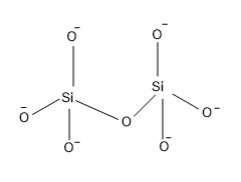
The structure of pyro silicate is,

So from the structure it is clear that, a Si atom which is surrounded by four O atoms having a negative charge that is the silicate tetrahedral structure of formulae $Si{{O}_{4}}^{4-}$ and two units of silicate units are combined at the corner of one of the O atom and in this combination one O is lost.

So the correct option for the answer is option (C).
Additional information:
On the basis of tetrahedral units of silicate combines, it is classified into:
Ortho silicates -$\left( Si{{O}_{4}}^{4-} \right)$
Pyro silicates - ($S{{i}_{2}}{{O}_{7}}^{6-}$)
Cyclic silicates-${{\left( Si{{O}_{3}} \right)}_{n}}^{2n-}$
Chain silicates-${{\left( Si{{O}_{3}} \right)}_{n}}^{2n-}$
Double chain silicates -${{\left( S{{i}_{4}}{{O}_{11}} \right)}_{n}}^{6-n}$
Sheet silicates-${{\left( S{{i}_{2}}{{O}_{5}} \right)}_{n}}^{2-n}$, with discrete structural units.
Note: Example of silicates is Thortveitite with the formulae, \[S{{c}_{2}}S{{i}_{2}}{{O}_{7}}\]. If instead of pyro silicate Thortveitite or if its formulae was give and asked to answer the structural unit of it then we may write, as Thortveitite comes under the classification of Pyro silicates the structural unit will be $S{{i}_{2}}{{O}_{7}}^{6-}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




