
The structure $I{{F}_{7}} $ is:
(A)- Square pyramid
(B)- Trigonal bipyramid
(C)- Octahedral
(D)- Pentagonal bipyramidal
Answer
577.8k+ views
Hint: An interhalogen compound with chemical formula $I{{F}_{7}}$ is known as Iodine heptafluoride or Iodine (VII) fluoride. The structure of iodine heptafluoride can be predicted using VSEPR theory.
Complete Step by step solution:
-An interhalogen compound with chemical formula $I{{F}_{7}}$ is known as Iodine heptafluoride or Iodine (VII) fluoride.
-The structure of iodine heptafluoride can be predicted using VSEPR theory.
-VSEPR theory is a strong and reliable theory for predicting the shape of many of the molecules from the electron pairs that surround the central atoms of the molecule. VSEPR is the abbreviation of Valence Shell Electron Pair Repulsion theory.
-VSEPR theory was first proposed by Sidgwick and Powell in the year 1940. This theory is based on the assumption that the molecule will take the shape such that the molecules experience minimum electronic repulsions.
-For predicting the shape of the molecule using VSEPR theory, the following steps must be followed-
(i) The least electronegative atom must be chosen as the central atom because this atom has the highest ability to share its electrons with the other atoms belonging to the molecule.
(ii) The total number of valence electrons, that is the electrons belonging to the outermost shell of the central atom must be counted.
(iii) The total number of bonded electrons, that is the electrons belonging to other atoms and which are used in the bond making must be counted.
(iv) The valence electrons of the central atom and the bonded electrons of the atoms must be added to obtain the valence shell electron pair number, which is abbreviated as VSEP number.
-The VSEP number determined will describe the molecule the shape of the molecule in the following way-
(i) If the VSEP number is 2, then the molecule is said to have a linear shape.
(ii) If the VSEP number is 3, then the molecule is said to have trigonal planar structure.
(iii) If the VSEP number is 4, then the molecule is said to have a tetrahedral structure.
(iv) If the VSEP number is 5, then the molecule is said to have trigonal bipyramidal structure.
(v) If the VSEP number is 6, then the molecule is said to have an octahedral structure.
(vi) If the VSEP number is 7, then the molecule is said to have pentagonal bipyramidal structure.
-Using the above guidelines, let us now try calculating the number of VSEP for predicting the structure of $I{{F}_{7}}$-
The least electronegative atom among iodine and fluorine is iodine, as the value of iodine is 2.66 and 3.98 for fluorine.
Number of electrons in the valence shell of the central atom = 7
Number of electrons provided by the 7 fluorine atoms $=7\times 1=7$
Total number of electrons around the central atoms $=7+7=14$
Therefore, the total number of electron pairs around the central atom$=\dfrac{14}{2}=7$
But, the total number of bond pairs = 7 (Because there are seven I-F bonds) = VSEP number
Therefore, the total number of lone pairs $=7-7=0$
So, according to VSEPR theory, the molecule will have pentagonal bipyramidal geometry.
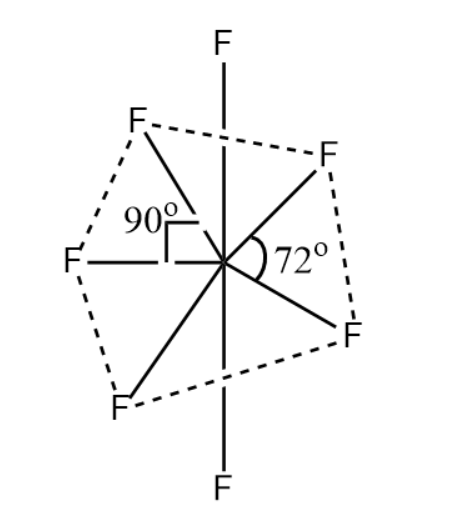
Hence, the correct answer is option D.
Note: However VSEPR theory has significant limitations too, which are as follows-
(i) VSEPR theory fails to explain about the structures of isoelectronic species (isoelectronic species are the elements having the same number of electrons). The isoelectronic species may have the same number of electrons but they may vary in shape.
(ii) The VSEPR theory didn’t say anything about compounds of transition metals. The structure of all transition metals was not correctly described by this theory because this theory does not take into account the associated size of the substituent groups and the lone pairs that are inactive.
(iii) According to VSEPR theory, the halides of group 2 elements will have a linear shape, but in actual they have a bent shape. So this was another failure of VSEPR theory.
Complete Step by step solution:
-An interhalogen compound with chemical formula $I{{F}_{7}}$ is known as Iodine heptafluoride or Iodine (VII) fluoride.
-The structure of iodine heptafluoride can be predicted using VSEPR theory.
-VSEPR theory is a strong and reliable theory for predicting the shape of many of the molecules from the electron pairs that surround the central atoms of the molecule. VSEPR is the abbreviation of Valence Shell Electron Pair Repulsion theory.
-VSEPR theory was first proposed by Sidgwick and Powell in the year 1940. This theory is based on the assumption that the molecule will take the shape such that the molecules experience minimum electronic repulsions.
-For predicting the shape of the molecule using VSEPR theory, the following steps must be followed-
(i) The least electronegative atom must be chosen as the central atom because this atom has the highest ability to share its electrons with the other atoms belonging to the molecule.
(ii) The total number of valence electrons, that is the electrons belonging to the outermost shell of the central atom must be counted.
(iii) The total number of bonded electrons, that is the electrons belonging to other atoms and which are used in the bond making must be counted.
(iv) The valence electrons of the central atom and the bonded electrons of the atoms must be added to obtain the valence shell electron pair number, which is abbreviated as VSEP number.
-The VSEP number determined will describe the molecule the shape of the molecule in the following way-
(i) If the VSEP number is 2, then the molecule is said to have a linear shape.
(ii) If the VSEP number is 3, then the molecule is said to have trigonal planar structure.
(iii) If the VSEP number is 4, then the molecule is said to have a tetrahedral structure.
(iv) If the VSEP number is 5, then the molecule is said to have trigonal bipyramidal structure.
(v) If the VSEP number is 6, then the molecule is said to have an octahedral structure.
(vi) If the VSEP number is 7, then the molecule is said to have pentagonal bipyramidal structure.
-Using the above guidelines, let us now try calculating the number of VSEP for predicting the structure of $I{{F}_{7}}$-
The least electronegative atom among iodine and fluorine is iodine, as the value of iodine is 2.66 and 3.98 for fluorine.
Number of electrons in the valence shell of the central atom = 7
Number of electrons provided by the 7 fluorine atoms $=7\times 1=7$
Total number of electrons around the central atoms $=7+7=14$
Therefore, the total number of electron pairs around the central atom$=\dfrac{14}{2}=7$
But, the total number of bond pairs = 7 (Because there are seven I-F bonds) = VSEP number
Therefore, the total number of lone pairs $=7-7=0$
So, according to VSEPR theory, the molecule will have pentagonal bipyramidal geometry.

Hence, the correct answer is option D.
Note: However VSEPR theory has significant limitations too, which are as follows-
(i) VSEPR theory fails to explain about the structures of isoelectronic species (isoelectronic species are the elements having the same number of electrons). The isoelectronic species may have the same number of electrons but they may vary in shape.
(ii) The VSEPR theory didn’t say anything about compounds of transition metals. The structure of all transition metals was not correctly described by this theory because this theory does not take into account the associated size of the substituent groups and the lone pairs that are inactive.
(iii) According to VSEPR theory, the halides of group 2 elements will have a linear shape, but in actual they have a bent shape. So this was another failure of VSEPR theory.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




