
What would be the structure of 4 - chloropentan - 2 - one?
Answer
570.3k+ views
Hint: As we know that nomenclature involves two systems for naming of the organic compounds and these are trivial systems and IUPAC systems. IUPAC system consists of a root or base, primary and secondary prefix and suffix and any substituent for naming a compound.
Complete step by step solution:
As we have learnt that IUPAC is the short for International Union of Pure and Applied Chemistry. It provides some rules for naming of the organic compounds. Using these rules we can write a unique name for any distinct compound and likewise we can make the structure from the given IUPAC name. IUPAC system consists of three main features which are: a root representing the chain of carbon atoms in molecular structure, presence of primary prefix, secondary prefix, primary suffix and secondary suffix and lastly the name of the substituents completing the molecular structure or formula.
Let us try and draw the correct structure of \[4 - chloropentan - 2 - one\]. We will go step by step as shown below:-
Step 1:- First we will find out the root word from the name given above, we can see that the root word is “pent” in the compound which means it has the longest chain of five carbon atoms.
$\mathop C\limits^5 - \mathop C\limits^4 - \mathop C\limits^3 - \mathop C\limits^2 - \mathop C\limits^1 $
Step 2:- Next we will find out any functional group attached, and we can see that there is one functional group attached that is chloro and it is numbered as $4$, hence we can make that it is attached on the fourth carbon atom.
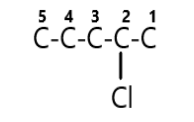
Step 3:- Next we will find out any ketone group attached, and yes there is one ketone group attached and it is numbered $2$, hence we can make that it is attached on the second carbon atom.
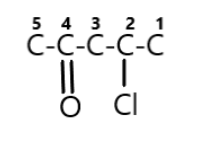
Lastly we can attach the remaining hydrogen atoms on the carbons. Therefore from the above steps we can say that the chemical formula for the \[4 - chloropentan - 2 - one\] is \[{C_5}{H_9}ClO\]. And its chemical structure is shown below as:
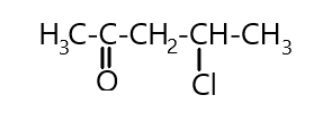
Note: Always remember that the parent chain will start from the side of attachment of the functional group. Also remember the order of preference of functional groups, direction of the numbering of carbon atoms and placing the halogen and ketone groups to their respective place and the bonding between carbon and oxygen as well as carbon and other atoms.
Complete step by step solution:
As we have learnt that IUPAC is the short for International Union of Pure and Applied Chemistry. It provides some rules for naming of the organic compounds. Using these rules we can write a unique name for any distinct compound and likewise we can make the structure from the given IUPAC name. IUPAC system consists of three main features which are: a root representing the chain of carbon atoms in molecular structure, presence of primary prefix, secondary prefix, primary suffix and secondary suffix and lastly the name of the substituents completing the molecular structure or formula.
Let us try and draw the correct structure of \[4 - chloropentan - 2 - one\]. We will go step by step as shown below:-
Step 1:- First we will find out the root word from the name given above, we can see that the root word is “pent” in the compound which means it has the longest chain of five carbon atoms.
$\mathop C\limits^5 - \mathop C\limits^4 - \mathop C\limits^3 - \mathop C\limits^2 - \mathop C\limits^1 $
Step 2:- Next we will find out any functional group attached, and we can see that there is one functional group attached that is chloro and it is numbered as $4$, hence we can make that it is attached on the fourth carbon atom.
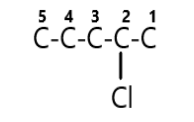
Step 3:- Next we will find out any ketone group attached, and yes there is one ketone group attached and it is numbered $2$, hence we can make that it is attached on the second carbon atom.
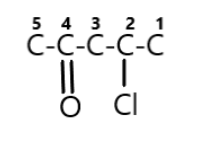
Lastly we can attach the remaining hydrogen atoms on the carbons. Therefore from the above steps we can say that the chemical formula for the \[4 - chloropentan - 2 - one\] is \[{C_5}{H_9}ClO\]. And its chemical structure is shown below as:
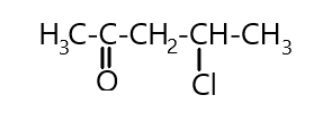
Note: Always remember that the parent chain will start from the side of attachment of the functional group. Also remember the order of preference of functional groups, direction of the numbering of carbon atoms and placing the halogen and ketone groups to their respective place and the bonding between carbon and oxygen as well as carbon and other atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




