
The structure of $C{{H}_{2}}=C{{H}_{2}}$ is
(A)- Linear
(B)- Planar
(C)- Non-planar
(D)- None of the above
Answer
595.2k+ views
Hint: The carbon atoms in $C{{H}_{2}}=C{{H}_{2}}$ are $s{{p}^{2}}$ hybridized. The hybridization of a molecule tells us about the geometry and shape of the molecule.
Complete step by step solution:
Let’s look at the answer
- The carbon atoms in the given molecule are both $s{{p}^{2}}$ hybridized.
-The shape of $s{{p}^{2}}$ hybridization is triangular planar.
-The carbon atom in the given molecule is linked to 2 sigma bonds to H atoms and one pi bond to another carbon atom.
-The pi bonds are formed by the lateral overlapping. For this the two atoms should be in the same plane for maximum overlap.
-In triangular planar geometry all the four atoms are in the same plane and the bond angles are$120{}^\circ $.
-The geometries of both the carbon atoms in the given molecule are triangular planar which is a planar geometry and the shape is also the same. Since, both the atoms are joined by double bonds so the molecule as a whole is planar.
Hence, the given molecule is a planar molecule.
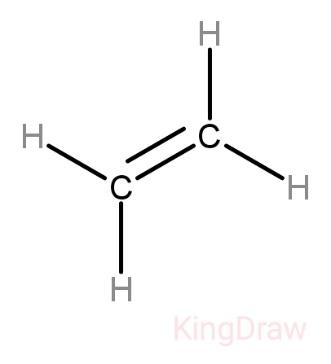
Structure of ethene
So, the correct answer is “Option B”.
Note: $C{{H}_{2}}=C{{H}_{2}}$is an alkene. In an alkene the single bond lengths and double bond lengths are different. It is also more acidic in nature than the corresponding alkane due to its geometry.
Complete step by step solution:
Let’s look at the answer
- The carbon atoms in the given molecule are both $s{{p}^{2}}$ hybridized.
-The shape of $s{{p}^{2}}$ hybridization is triangular planar.
-The carbon atom in the given molecule is linked to 2 sigma bonds to H atoms and one pi bond to another carbon atom.
-The pi bonds are formed by the lateral overlapping. For this the two atoms should be in the same plane for maximum overlap.
-In triangular planar geometry all the four atoms are in the same plane and the bond angles are$120{}^\circ $.
-The geometries of both the carbon atoms in the given molecule are triangular planar which is a planar geometry and the shape is also the same. Since, both the atoms are joined by double bonds so the molecule as a whole is planar.
Hence, the given molecule is a planar molecule.

Structure of ethene
So, the correct answer is “Option B”.
Note: $C{{H}_{2}}=C{{H}_{2}}$is an alkene. In an alkene the single bond lengths and double bond lengths are different. It is also more acidic in nature than the corresponding alkane due to its geometry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




